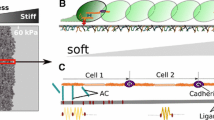
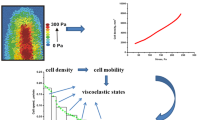
Abstract
Viscoelasticity of multicellular systems caused by collective cell migration depends on (1) viscoelasticity of migrating clusters, (2) viscoelasticity of surrounding resting cells and (3) the size, slip effects and thickness of the biointerface. A previously developed model for a sharp biointerface is expanded for the case of a finite biointerface based on thermodynamic and rheological considerations to estimate the influence of the biointerface properties on viscoelasticity. These properties of the interface layer are one of the key factors which influence the overall properties of the mixture, such as its viscoelasticity. Sliding of cell clusters through dense surroundings induces generation of significant shear stress, within the biointerface, which influences (1) the active (contractile) or passive state of single cells and (2) the state of cell–cell adhesion contacts. Cells retain collectivity in migration patterns even upon a reduction of cell–cell adhesion caused by stress generation. A greater size to the biointerface leads to the weakening of multicellular systems for the same volume fraction of migrating cells due to energy dissipation. Various factors such as (1) increase of the interface size, (2) reduction in slip effects under the constant thickness of the biointerface and (3) decrease in the biointerface thickness under constant slip effects induce an increase of the shear rate as well as the overall energy dissipation and can lead to circular cell movement within the biointerface layer. Additional experiments at subcellular and cellular levels are needed to determine the influence of mechanical factors on collective cell migration.




Similar content being viewed by others
References
Ala-Nissila T, Majaniemi K, Elder K (2004) Phase-field modelling of dynamical interface phenomena in fluids. Physics 640:357–388
Barriga EH, Mayor R (2019) Adjustable viscoelasticity allows for efficient collective cell migration. Sem Cell Dev Biol 93:55–68
Barriga EH, Franze K, Charras G, Mayor R (2018) Tissue stiffening coordinate morphogenesis by triggering collective cell migration in vivo. Nature 554:523–527
Blanchard GB, Fletcher AG, Schumacher LJ (2019) The devil is in the mesoscale: mechanical and behavioural heterogeneity in collective cell movement. Sem Cell Dev Biol 93:46–54
Campàs O, Mammoto T, Hasso S, Sperling RA, O’Connell D, Bischof AG, Maas R, Weitz DA, Mahadevan L, Ingber DE (2013) Quantifying cell-generated mechanical forces within living embryonic tissues. Nat Methods. https://doi.org/10.1038/nmMeth.2761
Clark AG, Vignjevic DM (2015) Models of cancer cell invasion and the rule of microenvironment. Cur Op Cell Biol 36:13–22
Cohen DS, Murray JD (1981) A generalized diffusion model for growth and dispersal in a population. J Mat Biol 12:237–249
Dolega ME, Delarue M, Ingremeau F, Prost J, Delon A, Cappello G (2017) Cell-like pressure sensors reveal increase of mechanical stress towards the core of multicellular spheroids under compression. Nature Comm 8(14056):1–9
Doxzen K, Vedula SRK, Leong MC, Hirata H, Gov NS, Kabla AJ, Ladoux B, Lim CT (2013) Guidance of collective migration by substrate geometry. Integr Biol 5:1026–1035
Du Roure O, Saez A, Buguin A, Austin RH, Chavrier P, Siberzan P, Ladoux B (2005) Force mapping in epithelial cell migration. PNAS 102(7):2390–2395
Flitney EW, Kuczmarski ER, Adam SA, Goldman RD (2009) Insights into the mechanical properties of epithelial cells: the effects of shear stress on the assembly and remodelling of keratin intermediate filaments. FASEB J 23(7):2110–2119
Friedl P, Alexander S (2011) Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147:992–1009
Friedl P, Mayor R (2017) Tuning collective cell migration by cell-cell junction regulation. Cold Spring Harb Perspect Biol 9:a029199
Hampel U, Garreis F, Burgemeister F, Eßel N, Paulsen F (2018) Effect of intermittent shear stress on corneal epithelial cells using an in vitro flow culture model. The Ocul Surf 16:341–351
Iyer KV, Gomez RP, Paijmans J, Julicher F, Eaton S (2019) Epithelial viscoelasticity is regulated by mechanosensitive e-cadherin turnover. Curr Biol 29:1–14
Kolarik J, Fambri L, Pegoretti A, Penati A (2000) Ternary polymer blends: prediction of mechanical properties for various phase structures. Polym Adv Technol 11:75–81
Kuwabara T, Parkins DG, Cogan DG (1976) Sliding of the epithelium in experimental corneal wounds. Inv Ophthalmol 15:4–14
Lange JR, Fabry B (2013) Cell and tissue mechanics in cell migration. Exp Cell Res 319:2418–2423
Liu Z, Tan JL, Cohen DM, Yang MT, Saidecki NJ, Ruiz SA, Nelson CM, Chen CS (2010) Mechanical tugging force regulates the size of cell–cell junctions. PNAS 107(22):9944–9949
Marmottant P, Mgharbel A, Kafer J, Audren B, Rieu JP, Vial JC, van der Sanden B, Maree AFM, Graner F, Delanoe-Ayari H (2009) The role of fluctuations and stress on the effective viscosity of cell aggregates. PNAS 106(41):17271–17275
Mikami T, Yoshida K, Sawada H, Esaki M, Yasumura K, Ono M (2015) Inhibition of Rho-associated kinases disturbs the collective cell migration of stratified TE-10 cells. Biol Res 48(1):1–15
Molladavoodi S, Robichaud M, Wulff D, Gorbet M (2017) Corneal epithelial cells exposed to shear stress show altered cytoskeleton and migratory behaviours. PLoS ONE 12(6):e0178981
Notbohm J, Banerjee S, Utuje KJC, Gweon B, Jang H, Park Y, Shin J, Butler JP, Fredberg JJ, Marchetti MC (2016) Cellular contraction and polarization drive collective cellular motion. Biophys J 110:2729–2738
Pajic-Lijakovic I, Milivojevic M (2017) Viscoelasticity of multicellular surfaces. J Biomech 60:1–8
Pajic-Lijakovic I, Milivojevic M (2019a) Long-time viscoelasticity of multicellular surfaces caused by collective cell migration—multi-scale modelling considerations. Sem Cell Dev Biol 93:87–96
Pajic-Lijakovic I, Milivojevic M (2019b) Functional epithelium remodelling in response to applied stress under in vitro conditions. Bionics Biomech Appl. https://doi.org/10.1155/2019/4892709
Petrungaro G, Morelli L, Uriu K (2019) Information flow in the presence of cell mixing and signalling delays during embryonic development. Sem Cell Dev Biol 93:26–35
Pitenis AA, Urueña JM, Hart SM, O’Bryan CS, Marshall SL, Levings PP, Angelini TE, Sawyer WG (2018) Friction-Induced Inflammation. Tribol Lett. https://doi.org/10.1007/s11249-018-1029-7
Ranft J, Basan M, Elgeti J, Joanny JF, Prost J, Julicher F (2010) Fluidization of tissues by cell division and apoptosis. PNAS 107(49):20863–20868
Shellard A, Mayor R (2019) Supracellular migration—beyond collective cell migration. J Cell Sci 132:jcs226142
Shi J, Barakat M, Chen D, Chen L (2018) Bicellular tight junctions and wound healing. Int J Mol Sci 19:3862. https://doi.org/10.3390/ijms19123862
Takayanagi M, Uemura S, Shunsuke M (1964) Application of equivalent model method to dynamic rheo-optical properties of crystalline polymer. J Polym Sci Part C 5:113–122
Tambe DT, Croutelle U, Trepat X, Park CY, Kim JH, Millet E, Butler JP, Fredberg JJ (2013) Monolayer stress microscopy: limitations, artifacts, and accuracy of recovered intercellular stresses. PLoS ONE 8(2):e55172
Tanaka H (1997) A Viscoelastic model of phase separation. Phys Rev 56(4):4451–4462
Zhang J, Chada NC, Reinhart-King CA (2019) Microscale interrogation of 3D tissue mechanics. Front Bioeng Biotechnol 7:412
Zhao T, Zhang Y, Wei Q, Shi X, Zhao P, Chen LQ, Zhang S (2018) Active cell-matrix coupling regulates cellular force landscapes of cohesive epithelial monolayers. Npj Comp Mat 10:1–6
Acknowledgements
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Contract No. 451-03-68/2020-14/200135).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author reports no conflict of interest. All authors contributed equally to the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
Model development for the sharp biointerface: formulation of the corresponding modulus ratio \(\frac{{G}_{T}^{*}\left(r,\omega \right)}{{G}_{r}^{*}\left(r,\omega \right)}\)
Complex modulus for the sharp biointerface \({G}_{T}^{*}\left(r,\omega \right)\) for steady condition (i.e. \({\upphi }_{m}\left(r\right)\) is time-independent) and parallel mode coupling is obtained based on the following steps:
Fourier transform of Eq. 5 can be expressed as:
Total stress and stresses of migrating and resting pseudo-phases from eq. 20 are obtained after Fourier transform of the corresponding constitutive model equations and expressed as:
Eqs 22 are introduced into eq. 20. Based on the strain equality described by eq. 21, the complex modulus \({G}_{T}^{*}\left(r,\omega \right)\) for the parallel mode coupling can be expressed as:
Complex modulus for the sharp biointerface \({G}_{T}^{*}\left(r,\omega \right)\) for steady condition (\(i.e. {\upphi }_{m}\left(r\right)\) is time-independent) and series mode coupling is obtained based on following steps:
Fourier transform of Eq. 6 can be expressed as:
Eqs 22 were introduced into eq. 25. Based on the stress equality described by eq. 24, the complex modulus \({G}_{T}^{*}\left(r,\omega \right)\) for the series mode coupling can be expressed as:
Appendix 2
Model development for the finite biointerface: formulation of the modulus ratio \(\frac{{G}_{m-\mathrm{i}\mathrm{n}\mathrm{t}}^{*}\left(r,\omega \right)}{{G}_{r}^{*}\left(r,\omega \right)}\)
Complex modulus for the finite biointerface \({G}_{m-\mathrm{i}\mathrm{n}\mathrm{t}}^{*}\left(r,\omega \right)\) for steady conditions (i.e. \({\upphi }_{m}\left(r\right)\) and \({\upphi }_{\mathrm{i}\mathrm{n}\mathrm{t}}\left(r\right)\) are time-independent) and parallel mode coupling is obtained based on the following steps:
Fourier transform of Eq. 11 can be expressed as:
where the volume fractions are \({\upphi }_{m}^{*}\left(r\right)=\frac{{\upphi }_{m}\left(r\right)}{1-{\upphi }_{r}\left(r\right)}\) and \({\upphi }_{\mathrm{i}\mathrm{n}\mathrm{t}}^{*}=\frac{{\upphi }_{\mathrm{i}\mathrm{n}\mathrm{t}}\left(r\right)}{1-{\upphi }_{r}\left(r\right)}\).
The stress \({\stackrel{\sim }{\sigma }}_{m-\mathrm{i}\mathrm{n}\mathrm{t}}\left(r,\omega \right)\), stress of migrating pseudo phase \({\stackrel{\sim }{\sigma }}_{m}\left(r,\omega \right)\) and stress of the interface \({\stackrel{\sim }{\sigma }}_{\mathrm{i}\mathrm{n}\mathrm{t}}\left(r,\omega \right)\) from eq. 27 are obtained after Fourier transform of the corresponding constitutive model equations and expressed as:
Eqs 29 are introduced into eq. 27. Based on the strain equality described by eq. 28, the complex modulus \({G}_{m-\mathrm{i}\mathrm{n}\mathrm{t}}^{*}\left(r,\omega \right)\) for the parallel mode coupling can be expressed by Eq. 15:
Appendix 3
Model development for the finite biointerface: formulation of the corresponding modulus ratio \(\frac{{G}_{T}^{*}\left(r,\omega \right)}{{G}_{r}^{*}\left(r,\omega \right)}\)
Complex modulus for the finite biointerface \({G}_{T}^{*}\left(r,\omega \right)\) for steady conditions (i.e. \({\upphi }_{m}\left(r\right)\) and \({\upphi }_{\mathrm{i}\mathrm{n}\mathrm{t}}\left(r\right)\) are time-independent) and parallel mode coupling is obtained based on the following steps:
Fourier transform of Eq. 12 can be expressed as:
Total stress and stresses of the pseudo-phases from eq. 31 are obtained after Fourier transform of the corresponding constitutive model equations and expressed as:
where the complex modulus \({G}_{m-\mathrm{i}\mathrm{n}\mathrm{t}}^{*}\left(r,\omega \right)\) is expressed by eq. 30 (Appendix 2).
Eqs 33 are introduced into eq. 31. Based on the strain equality described by eq. 32, the complex modulus \({G}_{T}^{*}\left(r,\omega \right)\) for the parallel mode coupling can be expressed as:
Complex modulus for the finite biointerface \({G}_{T}^{*}\left(r,\omega \right)\) for steady conditions and series mode coupling is obtained based on the following steps:
Fourier transform of Eq. 13 can be expressed as:
Eqs 33 were introduced into eq. 36. Based on the stress equality described by eq. 35, the complex modulus \({G}_{T}^{*}\left(r,\omega \right)\) for the series mode coupling can be expressed as:
Glossary of key terminology
- Collective and coordinated cell migration
-
Morphogenesis, wound healing, regeneration, and cancer invasion involve rearrangements of tissues which depend on the migration of large cell clusters. These clusters are closely connected and move in the same direction and at similar speeds to minimize energy dissipation and reach their target positions
- Stress accumulation caused by collective cell migration
-
Movement of cell clusters within dense surroundings made by (passive) “resting” cells induces generation of residual stress which has a feedback impact on configuration and volume fraction of migrating cells. Residual stress accumulation represents the consequence of cell–cell interactions at the biointerface between (active) migrating cells and (passive) resting cells
- Cell–cell adhesion contacts
-
Adherens junctions (AJs) and tight junctions (TJs) significantly influence cell polarization, signalling and migration. AJs are cadherin-catenin complexes linked to actin filaments. Cadherins are transmembrane glycoproteins containing an extracellular domain that mediates cell–cell adhesion via homophilic or heterophilic interactions and an intracellular domain that controls signalling cascades involved in a variety of cellular processes, including polarity, gene expression, etc. Mechanosensitivity of E-cadherin turnover depends on p120-catenin, a protein that binds to the E-cadherin tail and blocks access to the endocytic machinery. TJs are high-affinity complexes formed by transmembrane proteins, including claudins, occludins and tricellulins associate with numerous peripheral proteins. These complexes are also linked to actin filaments. AJs form relatively weak adhesion contacts compared with TJs
- Stiffness of migrating clusters
-
Transversal stiffness is a prerequisite of migrating clusters to keep their integrity during movement through dense surroundings. The stiffness is influenced by the state and types of adhesion contacts, the distribution of cell–cell adhesion contacts, as well as the establishment of some level of supracellular cytoskeletal organization
Rights and permissions
About this article
Cite this article
Pajic-Lijakovic, I., Milivojevic, M. Viscoelasticity of multicellular systems caused by collective cell migration: dynamics at the biointerface. Eur Biophys J 49, 253–265 (2020). https://doi.org/10.1007/s00249-020-01431-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-020-01431-2