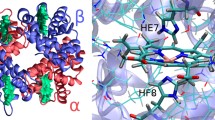
Abstract
Molecular dynamics simulations were applied to deoxy- and oxy-hemocyanins using newly developed force field parameters for the dicopper site to evaluate their structural and dynamical properties. Data obtained from the simulations provided information of the oxygenation effect on the active site and overall topology of the protein that was analyzed by root-mean-square deviations, b-factors, and dicopper coordination geometries. Domain I of the protein was found to demonstrate higher flexibility with respect to domain II because of the interfacial rotation between domain I and II that was further endorsed by computing correlative domain movements for both forms of the protein. The oxygenation effect on the overall structure of the protein or polypeptide subunit was further explored via gyration radii evaluated for the metal-binding domain and for the whole subunit. The evaluation of hydration dynamics was carried out to understand the water mediated role of amino acid residues of the solvent tunnel facilitating the entry of oxygen molecule to the dicopper site of hemocyanin.











Similar content being viewed by others

References
Ali SA, Grossmann JG, Abbasi A, Voelter W (2007) Structural and conformational analysis of scorpion (Buthus sindicus) hemocyanin using low resolution techniques. Protein Pept Lett 14(5):481–488
Babu CS, Lim C (2006) Empirical force fields for biologically active divalent metal cations in water. J Phys Chem A 110(2):691–699
Bernardi F, Bottoni A, Casadio R, Fariselli P, Rigo A (1996) Ab initio study of the mechanism of the binding of triplet O\(\_2\) to hemocyanin. Inorg Chem 35(18):5207–5212
Brown JM, Powers L, Kincaid B, Larrabee JA, Spiro TG (1980) Structural studies of the hemocyanin active site. 1. Extended X-ray absorption fine structure (EXAFS) analysis. J Am Chem Soc 102(12):4210–4216
Case DA, Betz RM, Cerutti DS, Cheatham TE III, Darden TA, Duke RE, Giese TJ, Gohlke H, Goetz AW, Homeyer N et al (2015) AMBER 2015. University of California, San Francisco
Coates CJ, Nairn J (2013) Hemocyanin-derived phenoloxidase activity: a contributing factor to hyperpigmentation in Nephrops norvegicus. Food Chem 140(1):361–369
Coates CJ, Nairn J (2014) Diverse immune functions of hemocyanins. Dev Comp Immunol 45(1):43–55
Cole DJ, Vilseck JZ, Tirado-Rives J, Payne MC, Jorgensen W (2016) Biomolecular force field parameterization via atoms in molecule electron density partitioning. J Chem Theory Comput 12(5):2312–2323
Cornell WD, Cieplak P, Bayly CI, Kollmann PA (1993) Application of RESP charges to calculate conformational energies, hydrogen bond energies, and free energies of solvation. J Am Chem Soc 115(21):9620–9631
Decker H, Tuczek F (2000) Tyrosinase/catecholoxidase activity of hemocyanins: structural basis and molecular mechanism. Trends Biochem Sci 25(8):392–397
Decker H, Van HKE (2010) Oxygen and the evolution of life. Springer Science and Business Media, Berlin
Dolashka P, Voelter W (2013) Antiviral activity of hemocyanins. Invertebr Surviv J 10:120–127
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) A smooth particle mesh Ewald method. J Chem Phys 103(19):8577–8593
Fariselli P, Bottoni A, Bernardi F, Casadio R (1999) Quantum mechanical analysis of oxygenated and deoxygenated states of hemocyanin: theoretical clues for a plausible allosteric model of oxygen binding. Protein Sci 8(07):1546–1550
Fiser A, Šali A (2003) Modeller: generation and refinement of homology-based protein structure models. Methods Enzymol 374:461–491
Fox T, Kollman PA (1998) Application of the RESP methodology in the parametrization of organic solvents. J Phys Chem B 102(41):8070–8079
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc., Wallingford
Glazer L, Tom M, Weil S, Roth Z, Khalaila I, Mittelman B, Sagi A (2013) Hemocyanin with phenoloxidase activity in the chitin matrix of the crayfish gastrolith. J Exp Biol 216(10):1898–1904
Grossmann JG, Ali SA, Abbasi A, Zaidi ZH, Stoeva S, Voelter W, Hasnain SS (2000) Low-resolution molecular structures of isolated functional units from arthropodan and molluscan hemocyanin. Biophys J 78(2):977–981
Hazes B, Hol WGJ (1992) Comparison of the hemocyanin \(\beta\)-barrel with other Greek key \(\beta\)-barrels: possible importance of the “\(\beta\)-zipper” in protein structure and folding. Proteins Struct Funct Bioinform 12(3):278–298
Hazes B, Kalk KH, Hol W, Magnus KA, Bonaventura C, Bonaventura J, Dauter Z (1993) Crystal structure of deoxygenated limulus polyphemus subunit II hemocyanin at 2.18 Å resolution: clues for a mechanism for allosteric regulation. Protein Sci 2(4):597–619
Hellmann N, Raithel K, Decker H (2003) A potential role for water in the modulation of oxygen-binding by tarantula hemocyanin. Comp Biochem Physiol A Mol Integr Physiol 136(3):725–734
Himmelwright RS, Eickman NC, LuBien CD, Solomon EI, Lerch K (1980) Chemical and spectroscopic studies of the binuclear copper active site of Neurospora tyrosinase: comparison to hemocyanins. J Am Chem Soc 102(24):7339–7344
Hofer TS, Tran HT, Schwenk CF, Rode BM (2004) Characterization of dynamics and reactivities of solvated ions by ab initio simulations. J Comput Chem 25(2):211–217
Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C (2006) Comparison of multiple force fields and development of improved protein backbone rarameters. Proteins Struct Funct Bioinform 65(3):712–725
Hu Z, Williams RD, Tran D, Spiro TG, Gorun SM (2000) Re-engineering enzyme-model active sites: reversible binding of dioxygen at ambient conditions by a bioinspired copper complex. J Am Chem Soc 122(14):3556–3557
Hundahl C, Fago A, Weber RE (2003) Effects of water activity on oxygen-binding in high-molecular weight, extracellular invertebrate hemoglobin and hemocyanin. Comp Biochem Physiol Part Biochem Mol Biol 136(1):83–90
Idakieva K, Meersman F, Gielens C (2012) Reversible heat inactivation of copper sites precedes thermal unfolding of Molluscan (Rapana thomasiana) hemocyanin. Biochemica et Biophysica Acta Proteins Proteom 1824(5):731–738
Jaenicke E, Pairet B, Hartmann H, Decker H (2012) Crystallization and preliminary analysis of crystals of the 24-meric hemocyanin of the emperor scorpion (Pandinus imperator). PLoS ONE 7(3):e32548
Kang KY, Scheraga AH (2008) An efficient method for calculating atomic charges of peptides and proteins from electronic populations. J Phys Chem B 112(17):5470–5478
Karplus M, McCammon JA (2002) Molecular dynamics simulations of biomolecules. Nat Struct Biol 9(9):646–652
Kay LE (1998) Protein dynamics from NMR. Nat Struct Biol 76(2):145–152
Kräutler V, Gunsteren WFV, Hünenberger PH (2001) A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J Comput Chem 22(5):501–508
Kuhn-Nentwig L, Kopp LS, Nentwig W, Haenni B, Streitberger K, Schürch S, Schaller J (2014) Functional differentiation of spider hemocytes by light and transmission electron microscopy, and MALDI-MS-imaging. Dev Comp Immunol 43(1):59–67
Lee AL, Wand AJ (2001) Nuclear magnetic resonance (NMR) spectroscopy for monitoring molecular dynamics in solution. eLS Essent Life Sci. https://doi.org/10.1038/npg.els.0003104
Lei K, Li F, Zhang M, Yang H, Luo T, Xu X (2008) Difference between hemocyanin subunits from shrimp Penaeus japonicus in anti-WSSV defense. Dev Comp Immunol 32(7):808–813
Lieb B, Gebauer W, Gatsogiannis C, Depoix F, Hellmann N, Harasewych MG, Strong EE, Markl J (2010) Molluscan mega-hemocyanin: an ancient oxygen carrier tuned by a 550 kDa polypeptide. Front Zool 7(1):1–13
Lindorff-Larsen K, Piana S, Palmo K, Maragakis P, Klepeis JL, Dror RO, Shaw DE (2010) Improved side-chain torsion potentials for the amber ff99SB protein force field. Proteins Struct Funct Bioinform 78(8):1950–1958
Ling J, Nestor LP, Czernuszewicz RS, Spiro TG, Fraczkiewicz R, Sharma KD, Loehr TM, Sanders-Loehr J (1994) Common oxygen binding site in hemocyanins from arthropods and mollusks. Evidence from Raman spectroscopy and normal coordinate analysis. J Am Chem Soc 116(17):7682–7691
Ma J (2005) Usefulness and limitations of normal mode analysis in modeling dynamics of biomolecular complexes. Structure 13(3):373–380
Magnus KA, Hazes B, Ton-That H, Bonaventura C, Bonaventura J, Hol WGJ (1994) Crystallographic analysis of oxygenated and deoxygenated states of arthropod hemocyanin shows unusual differences. Proteins Struct Funct Bioinform 19(4):302–309
Magnus KA, Ton-That H, Carpenter JE (1994) Recent structural work on the oxygen transport protein hemocyanin. Chem Rev 94(3):727–735
Markl J (2013) Evolution of molluscan hemocyanin structures. Biochemica et Biophysica Acta Proteins Proteom 1834(9):1840–1852
Mera-Adasme R, Sadeghian K, Sundholm D, Ochsenfeld C (2014) Effect of including torsional parameters for histidine–metal interactions in classical force fields for metalloproteins. J Phys Chem B 118(46):13106–13111
Moin ST, Hofer TS, Sattar R, Ul-Haq Z (2011) Molecular dynamics simulation of mammalian 15S-lipoxygenase with AMBER force field. Eur Biophys J 40(6):715–726
Naresh KN, Sreekumar A, Rajan S (2015) 43 studies using molecular dynamics reveal mechanism of interaction of hemocyanin with phenolic substrates. J Biomol Struct Dyn 33:29–30
Naresh K, Sreekumar A, Rajan SS (2015) Structural insights into the interaction between molluscan hemocyanins and phenolic substrates: an in silico study using docking and molecular dynamics. J Mol Graph Model 61:272–280
Neutze R (2014) Opportunities and challenges for time-resolved studies of protein structural dynamics at X-ray free-electron lasers. Phil Trans R Soc B 369(1647):20130318
Ohtaki H, Radnai T (1993) Structure and dynamics of hydrated ions. Chem Rev 93(3):1157–1204
Panzer D, Beck C, Hahn M, Maul J, Schönhense G, Decker H, Aziz EF (2010) Water influences on the copper active site in hemocyanin. J Phys Chem Lett 1(10):1642–1647
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612
Pick C, Hagner-Holler S, Burmester T (2008) Molecular characterization of hemocyanin and hexamerin from the firebrat Thermobia domestica (Zygentoma). 38(11):977–983
Roe DR, Cheatham TE III (2013) PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J Chem Theory Comput 9(7):3084–3095
Sagui C, Darden TA (1999) Molecular dynamics simulations of biomolecules: long-range electrostatic effects. Annu Rev Biophys Biomol Struct 28(1):155–179
Saito T, Thiel W (2014) Quantum mechanics/molecular mechanics study of oxygen binding in hemocyanin. J Phys Chem B 118(19):5034–5043
Sapienza PJ, Lee AL (2010) Using NMR to study fast dynamics in proteins: methods and applications. Curr Opin Pharmacol 10(6):723–730
Sigfridsson E, Ryde U (1998) Comparison of methods for deriving atomic charges from the electrostatic potential and moments. J Comput Chem 19(4):377–395
Singh UC, Kollman PA (1984) An approach to computing electrostatic charges for molecules. J Comput Chem 5(2):129–145
Skjærven L, Yao X, Scarabelli G, Grant BJ (2014) Integrating protein structural dynamics and evolutionary analysis with Bio3D. BMC Bioinform 15(1):399–410
Spinozzi F, Maccioni E, Teixeira CV, Amenitsch H, Favilla R, Goldoni M, Di MP, Salvato B, Mariani P, Beltramini M (2003) Synchrotron SAXS studies on the structural stability of Carcinus aestuarii hemocyanin in solution. Biophys J 85(4):2661–2672
Sterner R, Vogl T, Hinz H, Penz F, Hoff R, Föll R, Decker H (1995) Extreme thermostability of tarantula hemocyanin. FEBB Lett 364(1):9–12
Takano Y, Koizumi K, Nakamura H (2009) Theoretical studies of the magnetic couplings and the chemical indices of the biomimetic models of oxyhemocyanin and oxytyrosinase. Inorg Chimica Acta 362(12):4578–4584
Terwilliger NB (1998) Functional adaptations of oxygen-transport proteins. J Exp Biol 201(8):1085–1098
Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 25(9):1157–1174
Withers PC (1992) Comparative animal physiology. Saunders, Philadelphia
Yoon J, Fujii S, Solomon EI (2009) Geometric and electronic structure differences between the type 3 copper sites of the multicopper oxidases and hemocyanin/tyrosinase. Proc Natl Acad Sci 106(16):6585–6590
Zhang X, Huang C, Qin Q (2004) Antiviral properties of hemocyanin isolated from shrimp Penaeus monodon. Antivir Res 61(2):93–99
Zhang Y, Yan F, Hu Z, Zhao X, Min S, Du Z, Zhao S, Ye X, Li Y (2009) Hemocyanin from shrimp Litopenaeus vannamei shows hemolytic activity. Fish Shelfish Immunol 27(2):330–335
Zhuang J, Coates CJ, Zhu H, Zhu P, Wu Z, Xie L (2015) Identification of candidate antimicrobial peptides derived from abalone hemocyanin. Dev Comp Immunol 49(1):96–102
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bux, K., Ali, S.A. & Moin, S.T. Hydration facilitates oxygenation of hemocyanin: perspectives from molecular dynamics simulations. Eur Biophys J 47, 925–938 (2018). https://doi.org/10.1007/s00249-018-1316-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-018-1316-0