Abstract
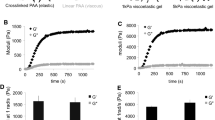
We used atomic force microscopy (AFM) technique to measure the viscoelastic response of cancer and normal thyroid cells on different stiffness polyacrylamide gels. After applying a step in contact we recorded the stress relaxation of cells in order to measure their viscous and elastic properties. With the help of an extended version of the Hertz model, we could quantify for the first time by AFM the elastic modulus and the dynamic viscosity of cells on substrates with different stiffnesses. We have cultured anaplastic carcinoma and normal thyroid cells on three different substrates: polyacrylamide gels with elastic modulus in a range of 3–5 and 30–40 kPa and “infinitely” stiff Petri dishes. Whereas normal thyroid cells adapted their mechanical properties to different stiffness substrates, cancer cells were less affected by the surrounding stiffness. Normal cells changed the elastic modulus from 1.2 to 1.6 and to 2.6 kPa with increasing substrate stiffness; the dynamic viscosity values varied from 230 to 515 and to 470 Pa·s, accordingly. By contrast, the values for cancer cells were rather constant regardless of substrate stiffness (in average the elastic modulus was 1.3 kPa and the dynamic viscosity was 300 Pa·s). This difference in sensing and reacting to the mechanical properties of the substrate shows that normal and cancer cells interact differently with the neighboring tissue, which may be related to the ability of cancer cells to form metastases.




Similar content being viewed by others
References
Ahmed WW, Fodor E, Almonacid M, Bussonnier M, Verlhac M-H, Gov NS, Visco P, van Wijland F, Betz T (2015) Active mechanics reveal molecular-scale force kinetics in living oocytes. arXiv:1510.08299
Alcaraz J, Buscemi L, Grabulosa M, Trepat X, Fabry B, Farré R, Navajas D (2003) Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys J 84:2071–2079
Aung A, Seo YN, Lu S, Wang Y, Jamora C, del Álamo JC, Varghese S (2014) 3D traction stresses activate protease-dependent invasion of cancer cells. Biophys J 107:2528–2537
Axelrod D, Koppel D, Schlessinger J, Elson E, Webb W (1976) Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J 16:1055
Braet F, Radmacher M (2012) Foreword to the special issue on AFM in biology and bionanomedicine. Micron 43:1211
Brooks SA, Lomax-Browne HJ, Carter TM, Kinch CE, Hall DM (2010) Molecular interactions in cancer cell metastasis. Acta Histochem 112:3–25
Cartagena A, Raman A (2014) Local viscoelastic properties of live cells investigated using dynamic and quasi-static atomic force microscopy methods. Biophys J 106:1033–1043
Chambers AF, Groom AC, MacDonald IC (2002) Metastasis: dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2:563–572
Coughlin MF, Fredberg JJ (2013) Changes in cytoskeletal dynamics and nonlinear rheology with metastatic ability in cancer cell lines. Phys Biol 10:065001
Cretu A, Castagnino P, Assoian R (2010) Studying the effects of matrix stiffness on cellular function using acrylamide-based hydrogels. J Vis Exp 42:2089
Dayel MJ, Hom EF, Verkman A (1999) Diffusion of green fluorescent protein in the aqueous-phase lumen of endoplasmic reticulum. Biophys J 76:2843–2851
Dembo M, Wang Y-L (1999) Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J 76:2307–2316
Discher DE, Janmey P, Y-l Wang (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310:1139–1143
Dunham W, Sands R, Klein S, Duell E, Rhodes L, Marcelo C (1996) EPR measurements showing that plasma membrane viscosity can vary from 30 to 100 cP in human epidermal cell strains. Spectrochim Acta A 52:1357–1368
Dvir L, Nissim R, Alvarez-Elizondo MB, Weihs D (2015) Quantitative measures to reveal coordinated cytoskeleton-nucleus reorganization during in vitro invasion of cancer cells. New J Phys 17:043010
Evans E, Yeung A (1989) Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J 56:151
Fidler IJ (1999) Critical determinants of cancer metastasis: rationale for therapy. Cancer Chemoth Pharm 43:S3–S10
Florin E-L, Radmacher M, Fleck B, Gaub HE (1994) Atomic force microscope with direct force modulation. Rev Sci Instrum 65:639–643
Fung YC (1993) Biomechanics - Mechanical properties of living tissues. Springer
Gal N, Weihs D (2012) Intracellular mechanics and activity of breast cancer cells correlate with metastatic potential. Cell Biochem Biophys 63:199–209
Haidekker MA, Ling T, Anglo M, Stevens HY, Frangos JA, Theodorakis EA (2001) New fluorescent probes for the measurement of cell membrane viscosity. Chem Biol 8:123–131
Hecht FM, Rheinlaender J, Schierbaum N, Goldmann WH, Fabry B, Schäffer TE (2015) Imaging viscoelastic properties of live cells by AFM: power-law rheology on the nanoscale. Soft Matter 11:4584–4591
Hertz H (1882) Über die Berührung fester elastischer Körper. J Reine Angew Mathematik 92:156–171
Hochmuth RM (2000) Micropipette aspiration of living cells. J Biomech 33:15–22
Hutter JL, Bechhoefer J (1993) Calibration of atomic-force microscope tips. Rev Sci Instrum 64:1868–1873
Iyer S, Gaikwad R, Subba-Rao V, Woodworth C, Sokolov I (2009) Atomic force microscopy detects differences in the surface brush of normal and cancerous cells. Nat Nanotechnol 4:389–393
Justin RT, Engler AJ (2011) Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS One 6(1):e15978
Kalwarczyk T, Ziebacz N, Bielejewska A, Zaboklicka E, Koynov K, Szymanski J, Wilk A, Patkowski A, Gapinski J, Butt Jr H (2011) Comparative analysis of viscosity of complex liquids and cytoplasm of mammalian cells at the nanoscale. Nano Lett 11:2157–2163
Kaufmann A, Heinemann F, Radmacher M, Stick R (2011) Amphibian oocyte nuclei expressing lamin A with the progeria mutation E145K exhibit an increased elastic modulus. Nucleus 2:310–319
Kausch I, Böhle A (2002) Molecular aspects of bladder cancer: III. Prognostic markers of bladder cancer. Eur Urol 41:15–29
Kollmannsberger P, Fabry B (2011) Linear and nonlinear rheology of living cells. Ann Rev Mater Res 41:75–97
Kristal-Muscal R, Dvir L, Weihs D (2013) Metastatic cancer cells tenaciously indent impenetrable, soft substrates. New J Phys 15:035022
Kristal-Muscal R, Dvir L, Ma Schvartzer, Weihs D (2015) Mechanical interaction of metastatic cancer cells with a soft gel. Procedia IUTAM 12:211–219
Kuimova MK (2012) Mapping viscosity in cells using molecular rotors. Phys Chem Chem Phys 14:12671–12686
Kuimova MK, Botchway SW, Parker AW, Balaz M, Collins HA, Anderson HL, Suhling K, Ogilby PR (2009) Imaging intracellular viscosity of a single cell during photoinduced cell death. Nat Chem 1:69–73
Lekka M, Laidler P (2009) Applicability of AFM in cancer detection. Nat Nanotechnol 4:72–73
Lekka M, Laidler P, Gil D, Lekki J, Stachura Z, Hrynmiewicz AZ (1999) Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. Eur Biophys J 28:312–316
Lekka M, Gil D, Pogoda K, Dulińska-Litewka J, Jach R, Gostek J, Klymenko O, Prauzner-Bechcicki S, Stachura Z, Wiltowska-Zuber J (2012a) Cancer cell detection in tissue sections using AFM. Arch Biochemi Biophys 518:151–156
Lekka M, Pogoda K, Gostek J, Klymenko O, Prauzner-Bechcicki S, Wiltowska-Zuber J, Jaczewska J, Lekki J, Stachura Z (2012b) Cancer cell recognition—mechanical phenotype. Micron 43:1259–1266
Li Q, Lee G, Ong C, Lim C (2008) AFM indentation study of breast cancer cells. Biochem Bioph Res Co 374:609–613
Lin HH, Lin HK, Lin IH, Chiou YW, Chen HW, Liu CY, Harn HIC, Chiu WT, Wang YK, Shen MR, Tang MJ (2015) Mechanical phenotype of cancer cells: cell softening and loss of stiffness sensing. Oncotarget 6:20946–20958
Luby-Phelps K (2000) Cytoarchitecture and physical properties of cytoplasm: volume, viscosity, diffusion, intracellular surface area. Int Rev Cyt 192:189–221
Margraves C, Kihm K, Yoon SY, Choi CK, Lee Sh, Liggett J, Baek SJ (2011) Simultaneous measurements of cytoplasmic viscosity and intracellular vesicle sizes for live human brain cancer cells. Biotechnol Bioeng 108:2504–2508
Martins-Green M, Bissell M (1995) Cell-ECM interactions in development. Semin Develop Biol 6:149–159
Mason TG, Gisler T, Kroy K, Frey E, Weitz DA (2000) Rheology of F-actin solutions determined from thermally driven tracer motion. J Rheol 44:917–928
Moreno-Flores S, Benitez R, Vivanco M, Toca-Herrera JL (2010a) Stress relaxation and creep on living cells with the atomic force microscope: a means to calculate elastic moduli and viscosities of cell components. Nanotechnology 21:445101
Moreno-Flores S, Benitez R, Vivanco M, Toca-Herrera JL (2010b) Stress relaxation microscopy: imaging local stress in cells. J Biomech 43:349–354
Needham D, Hochmuth R (1990) Rapid flow of passive neutrophils into a 4 μm pipet and measurement of cytoplasmic viscosity. J Biomech Eng 112:269–276
Nipper ME, Majd S, Mayer M, Lee JC-M, Theodorakis EA, Haidekker MA (2008) Characterization of changes in the viscosity of lipid membranes with the molecular rotor FCVJ. BBA-Biomembranes 1778:1148–1153
Planus E, Fodil R, Balland M, Isabey D (2002) Assessment of mechanical properties of adherent living cells by bead micromanipulation: comparison of magnetic twisting cytometry vs optical tweezers. J Biomech Eng 124:408–421
Pogoda K, Jaczewska J, Wiltowska-Zuber J, Klymenko O, Zuber K, Fornal M, Lekka A (2012) Depth-sensing analysis of cytoskeleton organization based on AFM data. Eur Biophys J 41:79–87
Prabhune M, Belge G, Dotzauer A, Bullerdiek J, Radmacher M (2012) Comparison of mechanical properties of normal and malignant thyroid cells. Micron 43:1267–1272
Radmacher M (1997) Measuring the elastic properties of biological samples with the atomic force microscope. IEEE Eng Med Bio 16:47–57
Rebelo LM, de Sousa JS, Mendes Filho J, Radmacher M (2013) Comparison of the viscoelastic properties of cells from different kidney cancer phenotypes measured with atomic force microscopy. Nanotechnology 24:055102
Rianna C, Radmacher M (2016) Cell mechanics as a marker for diseases: biomedical applications of AFM. AIP Conf Proc 1760:020057
Rico F, Roca-Cusachs P, Gavara N, Farré R, Rotger M, Navajas D (2005) Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys Review E 72:021914
Roan E, Wilhelm K, Bada A, Makena PS, Gorantla VK, Sinclair SE, Waters CM (2012) Hyperoxia alters the mechanical properties of alveolar epithelial cells. AM J Physiol-Lung C 302:L1235–L1241
Sen S, Kumar S (2012) Contributions of talin-1 glioma cell-matrix tensional homeostasis. J R Soc Interface 9:1311–1317
Sen S, Dong M, Kumar S (2009) Isoform-specific contributions of α-actin to glioma cell mechanobiology. PLoS ONE 4:e8427–e8427
Staunton JR, Doss BL, Lindsay S, Ros R (2016) Correlating confocal microscopy and atomic force indentation reveals metastatic cancer cells stiffen during invasion into collagen I matrices. Scientific Reports 6:19686
Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12:895–904
Sunyer R, Jin AJ, Nossal R, Sackett DL (2012) Fabrication of hydrogels with steep stiffness gradients for studying cell mechanical response. One 7:e46107
Suresh S (2007) Biomechanics and biophysics of cancer cells. Acta Mater 55:3989–4014
Thomas G, Burnham NA, Camesano TA, Wen Q (2013) Measuring the mechanical properties of living cells using atomic force microscopy. J Vis Exp 76:e50497
Tse JR, Engler AJ (2010) Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol Chapter 10:Unit 10 16
Ulrich TA, de Juan Pardo EM, Kumar S (2009) The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res 69:4167–4174
Ventre M, Netti PA (2016) Engineering cell instructive materials to control cell fate and functions through material cues and surface patterning. ACS Appl Mater Interfaces. doi:10.1021/acsami.5b08658
Ventre M, Causa F, Netti PA (2012) Determinants of cell–material crosstalk at the interface: towards engineering of cell instructive materials. J R Soc Interface 9:2017–2032
Wong SY, Ulrich TA, Deleyrolle LP, MacKay JL, Lin J-MG, Martuscello RT, Jundi MA, Reynolds BA, Kumar S (2015) Constitutive activation of myosin-dependent contractility sensitizes glioma tumor-initiating cells to mechanical inputs and reduces tissue invasion. Cancer Res 75:1113–1122
Woodhouse EC, Chuaqui RF, Liotta LA (1997) General mechanisms of metastasis. Cancer 80:1529–1537
Yamaguchi H, Condeelis J (2007) Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta 1773:642–652
Yango A, Schäpe J, Rianna C, Doschke H, Radmacher M (2016) Measuring the viscoelastic creep of soft samples by step response AFM. Soft Matter. doi:10.1039/C6SM00801A
Yarnell J, Baker IA, Sweetnam PM, Bainton D, O’Brien JR, Whitehead PJ, Elwood PC (1991) Fibrinogen, viscosity, and white blood cell count are major risk factors for ischemic heart disease. The Caerphilly and Speedwell collaborative heart disease studies. Circulation 83:836–844
Zhang H, Liu K-K (2008) Optical tweezers for single cells. J R Soc Interface 5:671–690
Acknowledgments
We thank Jörn Bullerdiek and Gazanfer Belge from the ZHG (University of Bremen) for providing cells. We thank Holger Doschke for developing the data acquisition and analysis software and Ken Jacobson for helpful discussions. AFM probes were a kind gift of Bruker, Santa Barbara, CA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Appendix
Appendix
Cell actin staining
Forty-eight hours after seeding on gels and Petri dishes, cells were fixed with 3.7 % formaldehyde for 15 min and then permeabilized with 0.1 % Triton X100 for 15 min. Samples were washed with PBS after each step and then incubated with a rhodamine phalloidin solution (5:200 dilution in PBS) for 30 min at 20 °C. Finally, cells were stored in PBS at 4 °C until image acquisition. An Axiovert 135 TV epifluorescence microscope (Carl Zeiss MicroImaging GmbH, Germany) was used to observe cells and collect fluorescent images (Fig. 5).
Fluorescent images of normal and cancer thyroidal cells labeled with rhodamine phalloidin (actin cytoskeleton). The upper panels show normal cells seeded on soft (a) and stiff (b) PA gels and on Petri dish (c). The lower panels show cancer cells, seeded on soft (d) and stiff (e) PA gels and on Petri dish (f). Scale bars are 50 μm
Analysis of stress relaxation data
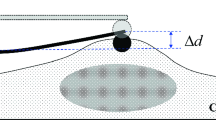
To analyze stress relaxation data, we assume that the sample can be described with the general linear solid model (Fig. 6).
Standard linear solid model. The sample is modeled by a Zener element, where a spring k 1 is in parallel to a Maxwell element, consisting of a spring k 2 and a viscous damping element f. The cantilever is characterized by its spring constant k c . Viscous (hydrodynamic) damping of the cantilever is neglected here, due to the slow stress relaxation of the sample, which is the predominant contribution
The force balance is given in the static case (directly after the step) by the following equation:
where k c is the force exerted by the cantilever, k1 and k2 is the forces exerted by the two springs. After relaxation F2 will be zero.
The force in the Maxwell element has to obey the following dynamic equation:
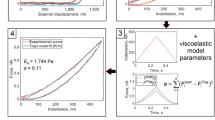
By fitting an exponential function (that was the more adequate to describe our results, see Fig. 7) to the deflection data, we determined the fit parameters ∆d, a, and τ.
In details, before applying the step the force equilibrium will be:
where z1, d1 and δ 1 are the z-position, the deflection and the indentation in contact with the sample, respectively. Since the forces are in equilibrium at this point, we can simplify our calculations such that:
When applying a z-step, the z-height will change to:
After relaxation, the deflection d 2 will be:
The spring k 2 will be relaxed due to the creep of the viscous damping element f, so the force balance looks like:
So, we can derive the spring constant k1 from the measurable quantities ∆d and ∆z:
After the step the force balance will be:
Therefore we can derive the spring constant k2
To describe the stress relaxation, we use the following relaxation process ansatz:
Therefore, Eq. (2) will change to:
From Eqs. (1), (11) and (12) we can get:
The time constant τ is not the true intrinsic relaxation time τ* of the Zener element, but the apparent relaxation time constant of the Zener element plus the cantilever spring. The intrinsic time constant τ* is given by:
Calculating elastic moduli and dynamic viscosity
When using pyramidal indenters, the elastic response is typically described by the Hertz model, which basically considers the increasing contact area when indentation increases.
In the case of a pyramidal tip with opening angle α, elastic modulus E and Poisson ratio ν, we find the following relation between indentation δ and loading force F:
The spring constant of a sample showing a Hertzian response can be found by taking the derivative. This force constant will depend on force F0 or indentation δ 0 at which it is calculated (or measured):
Since the indentation is not measured directly, but the deflection is, we use Eq. 15 to replace indentation by force, which is deflection times the cantilever’s force constant:
So, we can replace δ 0 in Eq. 16 and get the force constant as a function of the measurable quantity d 0, the deflection value at which the force constant is measured or calculated:
In a stress relaxation experiment, we actually measure the spring constants k1 and k2 of our sample, so we can invert Eq. 18 to convert spring constants in the corresponding moduli:
The dynamic viscosity η in the linear standard solid model can be written as:
where \(\tau^{*}\) is the intrinsic relaxation time as defined in Eq. 14.
Numerical results of cell moduli
Tables 1 and 2 show the numerical results presented in Fig. 3 in the main text for normal and cancer cells, respectively. Data are presented as median values and error bars show the 25th and 75th percentiles.
Statistical analysis of moduli between substrates
Differences between the moduli presented in Fig. 3 were determined with the Wilcoxon signed-rank test, calculated in IGOR, and considered significant when p < 0.05. We first calculated the median values of each force map, grouped them by cell type (normal and cancer) and substrate (soft and stiff gels, Petri dish), and checked whether the differences between groups were significant. Since the distribution of the data is not Gaussian, we could not refer to a t test, but had to use a Wilcoxon rank test, which has no assumptions on the distribution of data. We present here only significance values when comparing data from the same cell type on different substrates, since the hypothesis is that cancer and normal cells react differently on alterations of substrate stiffness. On a particular substrate, there may be (and actually are) differences also between the cell types, which we ignore here in the presentation. Significance data are presented as 2D matrixes, where each colored box presents one comparison (soft vs. stiff; soft vs. Petri, stiff vs. Petri). Since this matrix will be symmetrical, we plotted in the lower left corner the values for normal cells and in the upper right corner those for cancer cells. The significance values are color-coded such that all values below 0.05 show up as green, and all others as black. In this way, we can easily pick up differences between cancer and normal cells. Six matrixes are reported in Fig. 8 for each quantity presented in Fig. 3 (Young’s modulus from approach and retract, elastic modulus, and dynamic viscosity, each from loading and unloading steps).
Statistical analysis on Young’s moduli approach (a) and retract (b) from conventional force curves; elastic moduli E, loading (c) and unloading (d) and dynamic viscosity η, loading (e) and unloading (f) from stress relaxation experiments. In each matrix, cancer and normal cell cases are enclosed by red and blue lines, in the upper right and lower left corners, respectively. Differences are considered significant when p < 0.05 (green boxes) and not significant when p > 0.05 (black boxes)
It is apparent that for all quantities we have calculated, the values from normal cells are significantly different, virtually between all substrate combinations, whereas in the case of cancer cells the opposite behavior is true: virtually all quantities show non-significant differences between substrates.
Rights and permissions
About this article
Cite this article
Rianna, C., Radmacher, M. Comparison of viscoelastic properties of cancer and normal thyroid cells on different stiffness substrates. Eur Biophys J 46, 309–324 (2017). https://doi.org/10.1007/s00249-016-1168-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-016-1168-4