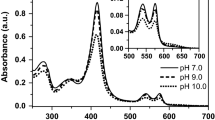
Abstract
Oxy-HbRa thermal stability was evaluated by dynamic light scattering (DLS) and small-angle X-ray scattering (SAXS) at pH 5.0, 7.0, 8.0, and 9.0. DLS results show that oxy-HbRa, at pH 7.0 and 5.0, remains stable up to 56 °C, undergoing denaturation/aggregation in acidic media above 60 °C, followed by partial sedimentation of aggregates. At alkaline pH values 8.0 and 9.0, oxy-HbRa oligomeric dissociation is observed above 30 °C, before denaturation. SAXS data show that oxy-HbRa, at 20 °C, is in its native form, displaying radius of gyration (R g) and particle maximum dimension (D max) of 108 ± 1 and 300 ± 10 Å, respectively. Oxy-HbRa, at pH 7.0, undergoes denaturation/aggregation at 60 °C. At pH 5.0–6.0, HbRa thermal denaturation/aggregation start earlier, at 50 °C, accompanied by an increase of R g and D max values. However, an overlap of oligomeric dissociation and denaturation in the system is observed upon temperature increase, with an increase in R g and D max. Analysis of experimental p(r) curves as a linear combination of theoretical curves obtained for HbGp fragments from the crystal structure shows an increasing contribution of dodecamer (abcd)3 and tetramer (abcd) in solution, as a function of pH values (8.0 and 9.0) and temperature. Finally, our data show, for the first time, that oxy-HbRa, in neutral and acidic media, does not undergo oligomeric dissociation before denaturation, while in alkaline media the oligomeric dissociation process is an important step in the thermal denaturation.






Similar content being viewed by others
References
Bachega JFR (2013) Estrutura cristalográfica da hemoglobina de Glossoscolex paulistus, um complexo de 3.6 mega Daltons (Crystallographic structure of Glossoscolex paulistus Hemoglobin, A complex of 3.6 MDa). Universidade de São Paulo, Instituto de Física de São Carlos (IFSC) (165 pp. Ph.D. Thesis (in Portuguese))
Bachega JFR, Bleicher L, Horjales ER, Santiago PS, Garratt RC, Tabak M (2011) Crystallization and preliminary structural analysis of the giant haemoglobin from Glossoscolex paulistus at 3.2 angstrom. J Synchrotron Radiat 18:2–28
Bachega JFR, Maluf FV, Andi B, Pereira HD, Carazzollea MF, Orville A, Tabak M, Garratt RC, Horjales E (2015) The crystallographic structure of the giant hemoglobin from Glossoscolex paulistus hemoglobin at 3.2 angstrom resolution. Acta Crystallogr D71:1257–1271. ID code 4U8U
Carvalho FAO, Carvalho JWP, Santiago PS, Tabak M (2011) Further characterization of the subunits of the giant extracellular hemoglobin of Glossoscolex paulistus (HbGp) by SDS-PAGE electrophoresis and MALDI-TOF-MS. Process Biochem 46:2144–2151
Carvalho JWP, Santiago PS, Batista T, Salmon CEG, Barbosa LRS, Itri R, Tabak M (2012) On the temperature stability of extracellular hemoglobin of Glossoscolex paulistus, at different oxidation states: SAXS and DLS studies. Biophys Chem 163–164:44–55
Carvalho JWP, Carvalho FAO, Santiago PS, Tabak M (2013) Thermal denaturation and aggregation of hemoglobin of Glossoscolex paulistus in acid and neutral media. Int J Biol Macromol 54:09–118
Carvalho FAO, Carvalho JWP, Biazin E, Santiago PS, Tabak M (2014a) Characterization of Rhinodrilus alatus hemoglobin (HbRa) and its subunits: evidence for strong interaction with cationic surfactants DTAB and CTAC. Comp Biochem Physiol Part B 167:23–29
Carvalho JWP, Carvalho FAO, Batista T, Santiago PS, Tabak M (2014b) Cetyltrimethylammonium chloride (CTAC) effect on the thermal stability of oxy-HbGp: dynamic light scattering (DLS) and small angle X-ray scattering (SAXS) studies. Colloids Surf B 118:14–24
Carvalho FAO, Alves FR, Carvalho JWP, Tabak M (2015) Guanidine hydrochloride and urea effects upon thermal stability of Glossoscolex paulistus hemoglobin (HbGp). Int J Biol Macromol 74:18–28
Desbruye ÁD, Laubier L (1982) Paralvinella grasslei, new genus, new species of Alvinellidae (Polycheata: ampharetidae) from the Galapagos Rift geothermal vents. Proc Biol Soc Wash 95:484–494
Glatter O, Kratky O (1982) Small angle X-ray scattering. Academic Press, London
Guinier A, Fournet G (1955) Small angle scattering of X-ray. Wiley, New York
Hirai M, Arai S, Iwase H (1999) Complementary analysis of thermal transition multiplicity of hen egg-white lysozyme at low pH using X-ray scattering and scanning calorimetry. J Phys Chem B 103:549–556
Jernshøj KD, Hassing S, Olsen LF (2013) A combination of dynamic light scattering and polarized resonance Raman scattering applied in the study of Arenicola Marina extracellular hemoglobin. J Chem Phys 139:139–142
Konarev PV, Petoukhov MV, Volkov VV, Svergun DI (2006) ATSAS 2.1, a program package for small-angle scattering data analysis. J Appl Cryst 39:277–286
Krebs A, Lamy J, Vinogradov SN, Zipper P (1998) Lumbricus terrestris hemoglobin: a comparison of small angle X-ray scattering and cryoelectron microscopy data. Biopolymers 45:289–298
Lamy JN, Green BN, Toulmond A, Wall JS, Weber RE, Vinogradov SN (1996) The giant haxagonal bilayer extracellular hemoglobins. Chem Rev 96:3113–3124
Mertens HDT, Svergun DI (2010) Structural characterization of proteins and complexes using small-angle X-ray solution scattering. J Struct Biol 172(1):128–141
Oliveira MS, Moreira LM, Tabak M (2007) Partial characterization of giant extracellular hemoglobin of Glossoscolex paulistus: a MALDI-TOF-MS study. Int J Biol Macromol 40:429–436
Pecora R (1985) Dynamic light scattering. Plenum Press, New York, pp 1–420
Poli AL, Moreira LM, Hidalgo AA, Imasato H (2005) Autoxidation studies of extracellular hemoglobin of Glossoscolex paulistus at pH 9: cyanide and hydroxyl effect. Biophys Chem 114:253–260
Putnam CD, Hammel M, Hura GL, Tainer JA (2007) X-ray solution scattering (SAXS) combined with crystallography and computation: defining accurate macromolecular structures, conformations and assemblies in solution. Q Rev Biophys 40:191–285
Rousselot M, Le Guen D, Chabasse C, Zal F (2006) Novel dissociation mechanism of a polychaetous annelid extracellular hemoglobin. FEBS J 273:1582–1596
Royer WE, Zhu H, Gorr TA, Flores JF, Knapp JE (2005) Allosteric hemoglobin assembly: diversity and similarity. J Biol Chem 280:27477–27480
Royer WE, Sharma H, Strand K, Knapp JE, Bhyravbhatla B (2006) Lumbricus erythrocruorin at 3.5 angstrom resolution: architecture of a megadalton respiratory complex. Structure 14:1167–1177
Santiago PS, Moreira LM, de Almeida EV, Tabak M (2007) Giant extracellular Glossoscolex paulistus Hemoglobin (HbGp) upon interaction with cetyltrimethylammonium chloride (CTAC) and sodium dodecyl sulphate (SDS) surfactants: dissociation of oligomeric structure and autoxidation. Biochim Biophys Acta 1770:506–517
Santiago PS, Moura F, Moreira LM, Domingues MM, Santos N, Tabak M (2008) Dynamic light scattering and optical absorption spectroscopy study of pH and temperature stabilities of the extracellular hemoglobin of Glossoscolex paulistus. Biophys J 94:2228–2240
Santiago PS, Carvalho FAO, Domingues MM, Carvalho JWP, Santos NC, Tabak M (2010a) Isoelectric point determination for Glossoscolex paulistus extracellular hemoglobin: oligomeric stability in acidic pH and relevance to protein-surfactant interactions. Langmuir 26:9794–9801
Santiago PS, Carvalho JWP, Domingues MM, Santos NC, Tabak M (2010b) Thermal stability of extracellular hemoglobin of Glossoscolex paulistus: determination of activation parameters by optical spectroscopic and differential scanning calorimetric studies. Biophys Chem 152:128–138
Svergun DI (1991) Mathematical methods in small-angle scattering data analysis. J Appl Cryst 24:485–492
Tabak M, Carvalho FAO, Carvalho JWP, Bachega JFR, Santiago PS (2012) Recent new characterizations on the giant extracellular hemoglobin of Glossoscolex paulistus and some other giant hemoglobins from different worms. In: Innocenti A (ed) Stoichiometry and research-the importance of quantity in biomedicine, vol 1. Intech Open Science, Croatia, pp 337–356
Vinogradov SN (2004) The stoichiometry of the four linker subunits of Lumbricus terrestris hemoglobin suggests an asymmetric distribution. Micron 35:127–129
Weber RE, Vinogradov SN (2001) Nonvertebrate hemoglobins: functions and molecular adaptations. Physiol Rev 81:570–611
Zal F, Rousselot M (2014) Extracellular hemoglobins from annelids, and their potential use in biotechnology. In: La Barre S, Jean-Michel K (eds) Outstanding marine molecules: chemistry, biology, analysis, 1st edn. Wiley, Weinheim, pp 361–376
Zal F, Green BN, Martineu P, Lallier FH, Toulmond A, Vinogradov SN, Childress JJ (2000) Polypeptide chain composition diversity of hexagonal-bilayer hemoglobins within a single family of annelids, the Alvinellidae. Eur J Biochem 267:5227–5236
Acknowledgments
The authors are grateful to the National Synchrotron Light Laboratory (LNLS), Campinas, Brazil, for making available the SAXS beam line used in these studies. Thanks are also due to the Brazilian agencies FAPESP, CNPq, and CAPES for partial financial support. F.A.O. Carvalho is a recipient of a post-doctoral grant from FAPESP (2013/09829-8). J.W.P. Carvalho is grateful for a post-doctoral grant from FAPESP (2013/09349-6) and M. Tabak is grateful to CNPq for a research grant. The authors are indebted to Prof. Dr. Carlos Ernesto Salmon Garrido, from Faculdade de Filosofia Ciências e Letras de Ribeirão Preto (FFCLRP), Universidade de São Paulo, for help with the analysis of linear combination of p(r) functions. The authors are also indebted to Dr. José Fernando Ruggiero Bachega for the help with the production of HbGp fragments from crystallographic structure used in the p(r) simulations as well as in Scheme 1. Thanks are also due to Mr. Ezer Biazin for efficient support in sample preparations.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carvalho, J.W.P., Carvalho, F.A.O., Santiago, P.S. et al. Thermal stability of extracellular hemoglobin of Rhinodrilus alatus (HbRa): DLS and SAXS studies. Eur Biophys J 45, 549–563 (2016). https://doi.org/10.1007/s00249-016-1121-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-016-1121-6