Abstract
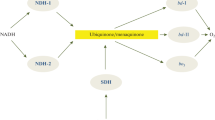
Cytochrome b561 (Cyt-b561) proteins constitute a family of trans-membrane proteins that are present in a wide variety of organisms. Two of their characteristic properties are the reducibility by ascorbate (ASC) and the presence of two distinct b-type hemes localized on two opposite sides of the membrane. Here we show that the tonoplast-localized and the putative tumor suppressor Cyt-b561 proteins can be reduced by other reductants than ASC and dithionite. A detailed spectral analysis of the ASC-dependent and dihydrolipoic acid (DHLA)-dependent reduction of these two Cyt-b561 proteins is also presented. Our results are discussed in relation to the known antioxidant capability of DHLA as well as its role in the regeneration of other antioxidant compounds of cells. These results allow us to speculate on new biological functions for the trans-membrane Cyt-b561 proteins.





Similar content being viewed by others
References
Asard H, Kapila J, Verelst W, Bérczi A (2001) Higher-plant plasma membrane cytochrome b561: a protein in search of a function. Protoplasma 217:77–93
Bast A, Haenen GRMM (1988) Interplay between lipoic acid and glutathione in the protection against microsomal lipid peroxidation. Biochim Biophys Acta 963:558–561
Bérczi A, Asard H (2006) Characterization of an ascorbate-reducible cytochrome b561 by site-directed mutagenesis. Acta Biol Szeged 52:257–265
Bérczi A, Asard H (2008) Expression and purification of the recombinant mouse tumor suppressor cytochrome b561 protein. Acta Biol Szeged 52:257–265
Bérczi A, Su D, Lakshminarasimhan M, Vargas A, Asard H (2005) Heterologous expression and site-directed mutagenesis of an ascorbate-reducible cytochrome b561. Arch Biochem Biophys 443:82–92
Bérczi A, Su D, Asard H (2007) An Arabidopsis cytochrome b561 with trans-membrane ferrireductase capability. FEBS Lett 581:1505–1508
Bérczi A, Desmet F, Van Doorslaer S, Asard H (2010) Spectral characterization of the recombinant mouse tumor suppressor 101F6 protein. Eur Biophys J 39:1129–1142
Biewenga GP, Haenen GRMM, Bast A (1997a) An overview of lipoate chemistry. In: Fuchs J, Packer L, Zimmer G (eds) Lipoic acid in health and disease. Marcell Dekker, New York, pp 1–32
Biewenga GP, Haenen GR, Bast A (1997b) The pharmacology of the antioxidant lipoic acid. Gen Pharmacol 29:315–331
Booker SJ (2004) Unraveling the pathway of lipoic acid biosynthesis. Chem Biol 11:10–12
Cenacchi L, Busch M, Schleidt PG, Müller FG, Stumpp TVM, Mäntele W, Trost P, Lancaster CRD (2012) Heterologous production and characterization of two distinct diheam-containing membrane integral cytochrome b561 enzymes from Arabidopsis thaliana in Pichia pastoris and Escherichia coli cells. Biochim Biophys Acta 1818:679–688
Desmet F, Bérczi A, Zimányi L, Asard H, Van Doorslaer S (2011) Axial ligation of the high-potential heme center in an Arabidopsis cytochrome b561. FEBS Lett 585:545–548
Flatmark T, Terland O (1971) Cytochrome b 561 of the bovine adrenal chromaffin granules. A high potential b-type cytochrome. Biochim Biophys Acta 253:487–491
Fleming PJ, Kent UM (1991) Cytochrome b561, ascorbic acid, and transmembrane electron transfer. Am J Clin Nutr 54:1173S–1178S
Glanfield A, McManus DP, Smyth DJ, Lovas EM, Loukas A, Gobert GF, Jones MK (2010) A cytochrome b561 with ferric reductase activity from the parasitic blood fluke, Schistosoma japonicum. PLoS Negl Tropic Dis 4:e884
Griesen D, Su D, Bérczi A, Asard H (2004) Localization of an ascorbate-reducible cytochrome b561 in the plant tonoplast. Plant Physiol 134:726–734
Han D, Hamilton RT, Lam PY, Packer L (2008) Modulation of cellular redox and metabolic status by lipoic acid. In: Petal MS, Packer L (eds) Lipoic acid. CRC Press, Boca Raton, pp 293–314
Henry ER, Hofrichter J (1992) Singular value decomposition: application to analysis of experimental data. Methods Enzymol 210:129–192
Herbert AA, Guest JR (1975) Lipoic acid content of Escherichia coli and other microorganisms. Arch Microbiol 106:259–266
Kagan VE, Shvedova A, Serbinova E, Khan S, Swanson C, Powell R, Packer L (1992) Dihydrolipoic acid—a universal antioxidant both in the membrane and in the aqueous phase. Reduction of peroxyl, ascorbyl and chromanoxyl radicals. Biochem Pharmacol 44:1637–1649
Kataoka H, Hirabayashi N, Makita M (1993) Analysis of lipoic acid in biological samples by gas chromatography with flame photometric detection. J Chromatog 615:197–202
Kelley PM, Njus D (1986) Cytochrome b561 spectral changes associated with electron transfer in chromaffin-vesicle ghosts. J Biol Chem 261:6429–6432
Kent UM, Fleming PJ (1987) Purified cytochrome b 561 catalyzes transmembrane electron transfer for dopamine β-hydroxylase and peptidyl glycine α-amidating monooxygenase activities in reconstituted systems. J Biol Chem 262:8174–8178
Kozlov AV, Gille L, Stanick K (1999) Dihydrolipoic acid maintains ubiquinone in the antioxidant active form by two-electron reduction of ubisemiquinone. Arch Biochem Biophys 363:148–154
Lakshminarasimhan M, Bérczi A, Asard H (2006) Substrate-dependent reduction of a recombinant chromaffin granule Cyt-b561 and its R72A mutant. Acta Biol Szeged 50:61–65
Li Y, Zhao Y, Yu W, Jiang S (2004) Scavenging ability on ROS of alpha-lipoic acid (ALA). Food Chem 84:563–567
Liu W, Kamensky Y, Kakkar R, Foley E, Kulmacz RJ, Palmer G (2005) Purification and characterization of bovine adrenal cytochrome b561 expressed in insect and yeast cell systems. Protein Expr Purif 40:429–439
Liu W, Rogge CE, Kamensky Y, Tsai A-L, Kulmacz RJ (2007) Development of a bacterial system for high yield expression of fully functional adrenal cytochrome b 561. Protein Expr Purif 56:145–152
Ludwiczek S, Rosell FI, Ludwiczek ML, Mauk AG (2008) Recombinant expression and initial characterization of the putative human enteric ferric reductase Dcytb. Biochemistry 47:753–761
Lykkesfeldt J, Hagen TM, Vladimir V, Ames BN (1998) Age-associated decline in ascorbic acid concentration, recycling, and biosynthesis in rat hepatocytes-reversal with (R)-α-lipoic acid supplementation. FASEB J 12:1183–1189
Markwell MAK, Haas SM, Bieber LL, Tolbert NE (1978) A modification of the Lowry procedure to simplify protein determinations in membrane and lipoprotein samples. Anal Biochem 87:206–210
McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A, Peters RJ, Raja KB, Shirali S, Hediger MA, Farzaneh F, Simpson RJ (2001) As iron-regulated ferric reductase associated with the absorption of dietary iron. Science 291:1755–1759
Mizutani A, Sanuki R, Kakimoto K, Kojo S, Taketani S (2007) Involvement of 101F6, a homologue of cytochrome b561, in the reduction of ferric ions. J Biochem 142:699–705
Nanasato Y, Akashi K, Yokota A (2005) Co-expression of cytochrome b561 and ascorbate oxidase in leaves of wild watermelon under drought and high light conditions. Plan Cell Physiol 46:1515–1524
Nichols TW (1997) Alpha-lipoic acid: biological effects and clinical implications. Alt Med Rev 2:177–183
Oakhill JS, Marritt SJ, Gareta EG, Cammack R, McKie AT (2008) Functional characterization of human duodenal cytochrome b (Cybrd1): Redox properties in relation to iron and ascorbate metabolism. Biochim Biophys Acta 1777:260–268
Okuyama E, Yamamoto R, Ichikawa Y, Tsubaki M (1998) Structural basis for the electron transfer across the chromaffin vesicle membranes catalyzed by cytochrome b 561: analysis of cDNA nucleotide sequences and visible absorption spectra. Biochim Biophys Acta 1383:269–278
Packer L, Witt EH, Tritschler HJ (1995) Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med 19:227–250
Packer L, Tritschler HJ, Wessel K (1996) Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med 22:359–378
Packer L, Roy S, Sen CK (1997) α-Lipoic acid: A metabolic antioxidant and potential redox modulator of transcription. In: Sies H (ed) Advances in pharmacology. Antioxidants in disease mechanisms and therapy, vol 18. Academic Press, San Diego, pp 79–101
Pérez-López U, Robredo A, Lacuesta M, Sgherri C, Mena-Petite A, Navari-Izzo F, Muñoz-Rueda A (2010) Lipoic acid and redox status in barley plants subjected to salinity and elevated CO2. Physiol Plant 139:256–268
Preger V, Pesaresi A, Pupillo P, Trost P (2005) Identification of an ascorbate-dependent cytochrome b of the tonoplast membrane sharing biochemical features with members of the cytochrome b561 family. Planta 220:365–375
Recuenco MC, Fujito M, Rahman M, Sakamoto Y, Takeuchi F, Tsubaki M (2009) Functional expression and characterization of human 101F6 protein, a homologue of cytochrome b561 and a candidate tumor suppressor gene product. BioFactors 34:219–230
Reed LJ (1974) Multienzyme complexes. Acc Chem Res 7:40–46
Scholich H, Murphy ME, Sies H (1989) Antioxidant activity of dihydrolipoate against microsomal lipid peroxidation and its dependence on α-tocopherol. Biochim Biophys Acta 1001:256–261
Sgherri C, Mike F, Quartacci MF, Izzo R, Navari-Izzo F (2002) Relation between lipoic acid and cell redox status in wheat grown in excess copper. Plant Physiol Biochem 40:591–597
Shih JCH, Steinsberger SC (1981) Determination of lipoic acid in chicken livers and chicken eggs during incubation. Anal Biochem 116:65–68
Shrager RI (1986) Chemical transition measured by spectra and resolved using singular value decomposition. Chemometr Intell Lab Syst 1:59–70
Srivastava M, Duong LT, Fleming PJ (1984) Cytochrome b561 catalyzes transmembrane electron transfer. J Biol Chem 259:8072–8075
Stokstad ELR, Seaman GR, Davis RJ, Hutner SH (1956) Assay of thioctic acid. In: Glick D (ed) Methods of biochemical analysis, vol 3. Wiley, New York, pp 23–47
Terland O, Flatmark T (1980) Oxidoreductase activities of chromaffin granule ghosts isolated from the bovine adrenal medulla. Biochim Biophys Acta 597:318–330
Tsubaki M, Nakayama M, Okuyama E, Ichikawa Y, Hori H (1997) Existence of two heme B centers in cytochrome b561 from bovine adrenal chromaffin vesicles as revealed by a new purification procedure and EPR spectroscopy. J Biol Chem 272:23206–23210
Tsubaki M, Takeuchi F, Nakanashi N (2005) Cytochrome b561 protein family: Expanding roles and versatile transmembrane electron transfer abilities as predicted by a new classification system and protein sequence motif analyses. Biochim Biophys Acta 1753:174–190
Tusnády GE, Simon I (1998) Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J Mol Biol 283:489–506
Tusnády GE, Simon I (2001) The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849–850
Verelst W, Asard H (2003) A phylogenetic study of cytochrome b561 proteins. Genome Biol 4:R38
Yasuno R, Wada H (1998) Biosynthesis of Lipoic Acid in Arabidopsis: Cloning and Characterization of the cDNA for Lipoic Acid Synthase. Plant Physiol 118:935–943
Zhang D, Su D, Bérczi A, Vargas A, Asard H (2006) An ascorbate-reducible cytochrome b561 is localized in macrophage lysosomes. Biochim Biophys Acta 1760:1903–1913
Zimányi L (2004) Analysis of the bacteriorhodopsin photocycle by singular value decomposition with self-modeling: a critical evaluation using realistic simulated data. J Phys Chem B 108:4199–4209
Acknowledgments
This work was supported by a grant from the Flemish Fund for Scientific Research (FWO; G.0118.07N) to H.A. and from the Hungary-Romania Cross Border Cooperation Program of the EU (HURO/0901/219) to A.B. and L.Z.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: Structure, function, folding and assembly of membrane proteins—Insight from Biophysics.
Rights and permissions
About this article
Cite this article
Bérczi, A., Zimányi, L. & Asard, H. Dihydrolipoic acid reduces cytochrome b561 proteins. Eur Biophys J 42, 159–168 (2013). https://doi.org/10.1007/s00249-012-0812-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-012-0812-x