Abstract
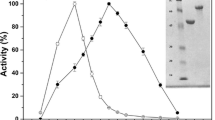
The folding of proteins in the milieu of the cellular environment involves various interactions among the residues of the polypeptide chain and the microenvironment where it resides. These interactions are responsible for stabilizing the protein molecule, and disruption of the same provides information about the stability of the molecule. β-Glucosidase isozymes, despite having high homology in their primary and tertiary designs, show deviations in their properties such as unfolding, refolding, and stability. In a comparative study on two large cell-wall-bound isozymes, β-glucosidase I (BGLI) and β-glucosidase II (BGLII) from a thermo-tolerant yeast, Pichia etchellsii, we have investigated guanidine hydrochloride (GdnHCl)-induced, alkali-induced, and thermal-unfolding transitions using CD and fluorescence spectroscopy and high sensitivity differential scanning calorimetry. Using spectral parameters (MRE 222 nm) to monitor the conformational transitions of the GdnHCl-induced unfolding phenomenon, it was observed that the midpoints of unfolding, apparent C m, occurred at 1.2 M ± 0.05 and 0.8 M ± 0.03 GdnHCl, respectively, for BGLI and BGLII. The alkali-induced unfolding process indicated that BGLI showed a mid-transition point at pH 11 ± 0.17, while for BGLII it was at pH 10 ± 0.40, further indicating BGLI to be more stable to alkali denaturation than BGLII. In the case of thermal unfolding, the midpoint of transition was observed at 63 ± 0.12°C for BGLI and at 58 ± 0.55°C for BGLII. Analysis by high sensitivity differential scanning calorimeter supported the unfolding data in which BGLI showed higher melting temperature, T m, (56.07°C ± 0.34) than BGLII (54.02°C ± 0.36). Our results clearly indicate that BGLI is structurally more rigid and stable than BGLII.













Similar content being viewed by others
Abbreviations
- BGLI:
-
β-Glucosidase I
- BGLII:
-
β-Glucosidase II
- C m :
-
Mid-point of transition
- C p :
-
Excess heat capacity
- DSC:
-
Differential scanning calorimetry
- GdnHCl:
-
Guanidine hydrochloride
- ∆H:
-
Enthalpy of denaturation
- MRE:
-
Molar residue ellipticity
- N and U:
-
Native and unfolded states, respectively
- T m :
-
Temperature of maximum heat capacity
References
Andrade MA, Chacón P, Merelo JJ, Morán F (1993) Evaluation of secondary structure of proteins from UV circular dichroism spectra using an unsupervised learning neural network. Protein Eng 6:383–390
Anfinsen CB (1973) Principles that govern the folding of protein chains. Science 181:223–230
Arakawaf T, Timasheff SN (1984) Protein stabilization and destabilization by guanidinium salts. Biochemistry 23:5924–5929
Bhatia Y, Mishra S, Bisaria VS (2002) Microbial β-glucosidases, cloning, properties and applications. Crit Rev Biotech 22:375–407
Burstein EA (1976) Luminescence of protein chromophores: model studies. In: Advances in science and technology. Series biophysics, vol 6. VINITI, Moscow
Cairoli S, Lametti S, Bonomi F (1994) Reversible and irreversible modification of beta-lactalbumin upon exposure to heat. J Protein Chem 13:347–354
Czjzek M, Cicek M, Zamboni V, Burmeister WP, Bevan RD, Henrissat B, Esen A (2001) Crystal structure of a monocotyledon (maize ZMGlu1) beta-glucosidase and a model of its complex with p-nitrophenyl β-D-thioglucoside. Biochem J 354:37–46
Fitter J, Haber-Pohlmeier S (2004) Structural stability and unfolding properties of thermo-stable bacterial amylases: a comparative study of homologous enzymes. Biochemistry 43:9589–9599
Freifelder D (1982) Physical biochemisrty, appplications to biochemistry and molecular biology, 2nd edn. W.H. Freeman, New York
Hakulinen N, Paavilainen S, Korpela T, Rouvinen J (2000) The crystal structure of beta-glucosidase from Bacillus circulans sp. alkalophilus: ability to form long polymeric assemblies. J Struct Bio 129:69–79
Halfman CJ, Nishida T (1971) Influence of pH and electrolyte on the fluorescence of bovine serum albumin. Biochim Biophys Acta 243:284–293
Hayashi S, Nakamura S (1981) Multiple forms of glucose oxidase with different carbohydrate compositions. Biochim Biophys Acta 657:40–51
Henrissat B, Davies GJ (1997) Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol 7:637–644
Kaper T, Lebbink JH, Pouwels J, Kopp J, Schulz GE, van der Oost J, de Vos WM (2000) Comparative structural analysis and substrate specificity engineering of the hyperthermostable β-glucosidase CelB from Pyrococcus furiosus. Biochemistry 39:4963–4970
Kuwajima K (1996) The molten globule state of α-lactalbumin. FASEB J 10:102–109
Masuko T, Miami A, Iwasaki N, Majima T, Nshimura S, Lee YC (2005) Carbohydrate analysis by a phenol-sulphuric acid in microplate format. Anal Biochem 339:69–72
Plum GE, Breslauer KJ (1995) Calorimetry of proteins and nucleic acids. Curr Opin Struct Biol 5:682–690
Ptitsyn OB (1992) The molten globule state. In: Creighton TE (ed) Protein folding. W.H. Freeman, New York, pp 243–300
Regan L (2003) Molten globules move into action. Proc Natl Acad Sci USA 100:3553–3554
Struksberg KH, Rosenkranz T, Fitter J (2007) Reversible and irreversible unfolding of multi-domain proteins. Biochem Biophys Acta 1774:1591–1603
Timasheff SN (1993) The control of protein stability and association by weak interactions in water: how do solvents affect these processes? Annu Rev Biophys Biomol Struct 22:67–97
Tolley SP, Barnett TE, Suresh CG, Hughes MA (1993) Crystallization and preliminary crystallographic analysis of the cyanogenic β-glucosidase from the white clover Trifolium repens. J Mol Biol 229:791–793
Varghese JN, Hrmova M, Fincher GB (1999) Three dimensional structure of barley beta-D-glucan exohydrolase, a family 3 glycosylhydrolase. Struct Fold Des 7:179–190
Wallecha A, Mishra S (2003) Purification and characterization of two β-glucosidases from a thermo- tolerant yeast Pichia etchellsii. Biochim Biophys Acta 1649:74–84
Wiesmann C, Beste G, Hengstenberg W, Schulz GE (1997) Three dimensional structure of 6-phosphor β-galactosidase from Lactococcus lactis. Structure 3:961–968
Yoshida E, Hidaka M, Fushinobu S, Koyanagi T, Minami H, Tamaki H, Kitaoka M, Katayama T, Kumagai H (2010) Purification, crystallization and preliminary x-ray analysis of β-glucosidase from Kluyveromyces marxianus NBRC 1777. Biochem J 431:39–49
Acknowledgments
The Department of Biotechnology (Govt. of India) is acknowledged for providing financial support to one of the authors (S.M.) for carrying out this work. The support staff at Advanced Instrumentation Facility, Jawaharlal Nehru University, New Delhi is acknowledged for providing the Chirascan CD polarimeter facility. The authors acknowledge the Varian spectrofluorometer facility provided by Dr. Shashank Deep from the Department of Chemistry, Indian Institute of Technology Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, M.A., Mishra, S. & Chaudhuri, T.K. Structural stability and unfolding transition of β-glucosidases: a comparative investigation on isozymes from a thermo-tolerant yeast. Eur Biophys J 40, 877–889 (2011). https://doi.org/10.1007/s00249-011-0706-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-011-0706-3