Abstract
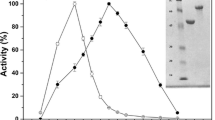
Psychrophilic enzymes display efficient activity at moderate or low temperatures (4–25 °C) and are therefore of great interest in biotechnological industries. We previously examined the crystal structure of BglU, a psychrophilic β-glucosidase from the bacterium Micrococcus antarcticus, at 2.2 Å resolution. In structural comparison and sequence alignment with mesophilic (BglB) and thermophilic (GlyTn) counterpart enzymes, BglU showed much lower contents of Pro residue and of charged amino acids (particularly positively charged) on the accessible surface area. In the present study, we investigated the roles of specific amino acid residues in the cold adaptedness of BglU. Mutagenesis assays showed that the mutations G261R and Q448P increased optimal temperature (from 25 to 40–45 °C) at the expense of low-temperature activity, but had no notable effects on maximal activity or heat lability. Mutations A368P, T383P, and A389E significantly increased optimal temperature (from 25 to 35–40 °C) and maximal activity (~1.5-fold relative to BglU). Thermostability of A368P and A389E increased slightly at 30 °C. Mutations K163P, N228P, and H301A greatly reduced enzymatic activity—almost completely in the case of H301A. Low contents of Pro, Arg, and Glu are important factors contributing to BglU’s psychrophilic properties. Our findings will be useful in structure-based engineering of psychrophilic enzymes and in production of mutants suitable for a variety of industrial processes (e.g., food production, sewage treatment) at cold or moderate temperatures.






Similar content being viewed by others
References
Badhan AK, Chadha BS, Kaur J, Saini HS, Bhat MK (2007) Production of multiple xylanolytic and cellulolytic enzymes by thermophilic fungus Myceliophthora sp. IMI 387099. Bioresour Technol 98:504–510
Bhatia Y, Mishra S, Bisaria VS (2002) Microbial β-glucosidases: cloning, properties, and applications. Crit Rev Biotechnol 22:375–407
Choo DW, Kurihara T, Suzuki T, Soda K, Esaki N (1998) A cold-adapted lipase of an Alaskan psychrotroph, Pseudomonas sp. strain B11-1: gene cloning and enzyme purification and characterization. Appl Environ Microbiol 64:486–491
Chuenchor W, Pengthaisong S, Robinson RC, Yuvaniyama J, Oonanant W, Bevan DR, Esen A, Chen CJ, Opassiri R, Svasti J, Cairns JR (2008) Structural insights into rice BGlu1 β-glucosidase oligosaccharide hydrolysis and transglycosylation. J Mol Biol 377:1200–1215
Eyles SJ, Gierasch LM (2000) Mutiple roles of prolyl residues in structure and folding. J Mol Biol 301:737–747
Fan H-X, Miao L-L, Liu Y, Liu H-C, Liu Z-P (2011) Gene cloning and characterization of a cold-adapted β-glucosidase belonging to glycosyl hydrolase family 1 from a psychrotolerant bacterium Micrococcus antarcticus. Enzyme Microbial Technol 49:94–99
Faure D (2002) The family-3 glycoside hydrolases: from housekeeping functions to host-microbe interactions. Appl Environ Microbiol 68:1485–1490
Feller G (2013) Psychrophilic enzymes: from folding to function and biotechnology. Scientifica 2013:512840
Gerday C, Aittaleb M, Bentahir M, Chessa JP, Claverie P, Collins T, D’Amico S, Dumont J, Garsoux G, Georlette D, Hoyoux A, Lonhienne T, Meuwis MA, Feller G (2000) Cold-adapted enzymes: from fundamentals to biotechnology. Trends in Biotechnol 18:103–107
Hong J, Tamaki H, Kumagai H (2007) Cloning and functional expression of thermostable β-glucosidase gene from Thermoascus aurantiacus. Appl Microbiol Biotechnol 73:1331–1339
Hong MR, Kim YS, Park CS, Lee JK, Kim YS, Oh DK (2009) Characterization of a recombinant β-glucosidase from the thermophilic bacterium Caldicellulosiruptor saccharolyticus. J Biosci Bioeng 108:36–40
Isorna P, Polaina J, Latorre-Garcia L, Canada FJ, Gonzalez B, Sanz-Aparicio J (2007) Crystal structures of Paenibacillus polymyxa β-glucosidase B complexes reveal the molecular basis of substrate specificity and give new insights into the catalytic machinery of family I glycosidases. J Mol Bio 371:1204–1218
Jónsdóttir LB, Ellertsson BÖ, Invernizzi G, Magnúsdóttir M, Thorbjarnardóttir SH, Papaleo E, Kristjánsson MM (2014) The role of salt bridges on the temperature adaptation of aqulysin I, a thermostable subtilisin-like proteinase. Biochim Biophys Acta 1844:2174–2181
Kane L, Mindy M, Vincent JJM (2016) Directed evolution of a fungal beta glucosidase in Saccharomyces cerevisiae. Biotech Biofuels 9:52
Laidler KJ, King MC (1983) The development of transition-state theory. J Phys Chem 87(15):2657–2664
Liu H, Xu Y, Ma Y, Zhou P (2000) Characterization of Micrococcus antarcticus sp. nov., a psychrophilic bacterium from Antarctica. Int J Syst Evol Microbiol 50:715–719
Miao L-L, Hou Y-J, Fan H-X, Qu J, Qi C, Liu Y, Li D-F, Liu Z-P (2016) Molecular structural basis for the cold adaptedness of the psychrophilic β-glucosidase BglU in Micrococcus antarcticus. Appl Environ Microbiol 82(7):2021–2030
Montella S, Balan V, da Costa SL, Gunawan C, Giacobbe S, Pepe O, Faraco V (2016) Saccharification of newspaper waste after ammonia fiber expansion or extractive ammonia. AMB Express 6(1):18
Neil D, Rawlings GS (2013) Handbook of proteolytic enzymes. Academic Press, New York Chapter 695
Ramli ANM, Azhar MA, Shamsir MS, Rabu A, Murad AMA, Mahadi NM, Illias Md R (2013) Sequence and structural investigation of a novel psychrophilic α-amylase from Glaciozyma antarctica PI12 for cold-adaptation analysis. J Mol Model 19:3369–3383
Siddiqui KS (2015) Some like it hot, some like it cold: temperature dependent biotechnological applications and improvements in extremophilic enzymes. Biotechnol Adv 33:1912–1922
Siddiqui KS, Poljak A, Guilhaus M, De Francisci D, Curmi PM, Feller G, D’Amico S, Gerday C, Uversky VN, Cavicchioli R (2006) Role of lysine versus arginine in enzyme cold-adaptation: modifying lysine to homo-arginine stabilizes the cold-adapted alpha-amylase from Pseudoalteramonas haloplanktis. Proteins 64:486–501
Wallecha A, Mishra S (2003) Purification and characterization of two β-glucosidases from a thermo-tolerant yeast Pichia etchellsii. Biochim Biophys Acta 1649:74–84
Wang X, He X, Yang S, An X, Chang W, Liang D (2003) Structural basis for thermostability of β-glycosidase from the thermophilic eubacterium Thermus nonproteolyticus HG102. J Bacteriol 185:4248–4255
Wolterink-van Loo S, Siemerink MA, Perrakis G, Kaper T, Kengen SW, van der Oost J (2009) Improving low-temperature activity of Sulfolobus acidocaldarius 2-keto-3-deoxygluconate aldolase. Archaea 2:233–239
Yeom SJ, Kim BN, Kim YS, Oh DK (2012) Hydrolysis of isoflavone glycosides by a thermostable β-glucosidase from Pyrococcus furiosus. J Agric Food Chem 60:1535–1541
Zanphorlin LM, de Giuseppe PO, Honorato RV, Costa Tonoli CC, Fattori J, Crespim E, de Oliveira PS, Ruller R, Murakami MT (2016) Oligomerization as a strategy for cold adaptation: structure and dynamics of the GH1 β-glucosidase from Exiguobacterium antarcticum B7. Sci Rep 6:23776–23789
Acknowledgments
This study was supported by grants from the National Nature Science Foundation of China (No. 30970102) and the Knowledge Innovation Program of the Chinese Academy of Sciences (No. KSCS2-YW-G-055-01). The authors are grateful to Dr. S. Anderson for the English editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Miao, LL., Fan, HX., Qu, J. et al. Specific amino acids responsible for the cold adaptedness of Micrococcus antarcticus β-glucosidase BglU. Appl Microbiol Biotechnol 101, 2033–2041 (2017). https://doi.org/10.1007/s00253-016-7990-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7990-x