Abstract
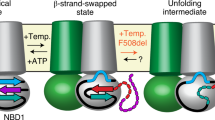
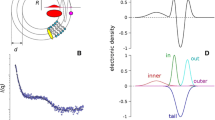
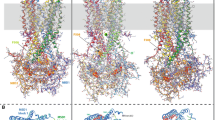
Nucleotide binding domains (NBD1 and NBD2) of the cystic fibrosis transmembrane conductance (CFTR), the defective protein in cystic fibrosis, are responsible for controlling the gating of the chloride channel and are the putative binding site for several candidate drugs in the disease treatment. We studied the structural properties of recombinant NBD1, NBD2, and an equimolar NBD1/NBD2 mixture in solution by small-angle X-ray scattering. We demonstrated that NBD1 or NBD2 alone have an overall structure similar to that observed for crystals. Application of 2 mM ATP induces a dimerization of NBD1 but does not modify the NBD2 monomeric conformation. An equimolar mixture of NBD1/NBD2 in solution shows a dimeric conformation, and the application of ATP to the solution causes a conformational change in the NBD1/NBD2 complex into a tight heterodimer. We hypothesize that a similar conformation change occurs in situ and that transition is part of the gating mechanism. To our knowledge, this is the first direct observation of a conformational change of the NBD1/NBD2 interaction by ATP. This information may be useful to understand the physiopathology of cystic fibrosis.










Similar content being viewed by others
References
Annereau JP, Wulbrand U, Vankeerberghen A, Cuppens H, Bontems F, Tümmler B, Cassiman JJ, Stoven V (1997) A novel model for the first nucleotide binding domain of the cystic fibrosis transmembrane conductance regulator. FEBS Lett 407:303–308
Awayn NH, Rosenberg MF, Kamis AB, Aleksandrov LA, Riordan JR, Ford RC (2005) Crystallographic and single-particle analyses of native- and nucleotide-bound forms of the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Biochem Soc Trans 33:996–999
Csanády L, Nairn AC, Gadsby DC (2006) Thermodynamics of CFTR channel gating: a spreading conformational change initiates an irreversible gating cycle. J Gen Physiol 128:523–533
Feigin LA, Svergun DI (1987) Structure analysis by small-angle X-ray and neutron scattering. Plenum Press, New York
Franke D, Svergun DI (2009) DAMMIF, a program for rapid ab initio shape determination in small-angle scattering. J Appl Cryst 42:342–346
Gadsby DC, Vergani P, Csanády L (2006) The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature 440:477–483
Glatter O, Kratky O (1982) Small angle X-ray scattering. Academic Press, London
Guinier A, Fournet G (1955) Small angle scattering of X-rays. Wiley, New York
Hartman J, Huang Z, Rado TA, Peng S, Jilling T, Muccio DD, Sorscher EJ (1992) Recombinant synthesis, purification, and nucleotide binding characteristics of the first nucleotide binding domain of the cystic fibrosis gene product. J Biol Chem 267:6455–6458
Karpowich N, Martsinkevich O, Millen L, Yuan YR, Dai PL, MacVey K, Thomas PJ, Hunt JF (2001) Crystal structures of the MJ1267 ATP binding cassette reveal an induced-fit effect at the ATPase active site of an ABC transporter. Structure 9:571–586
Kidd JF, Ramjeesingh M, Stratford F, Huan LJ, Bear CE (2004) A heteromeric complex of the two nucleotide binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) mediates ATPase activity. J Biol Chem 279:41664–41669
Konarev P, Volkov VV, Sokolova AV, Koch MHJ, Svergun DI (2003) PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J Appl Cryst 36:1277–1282
Kozin MB, Svergun DI (2001) Automated matching of high- and low-resolution structural models. J Appl Cryst 34:33–41
Lewis HA, Zhao X, Wang C, Sauder JM, Rooney I, Noland BW, Lorimer D, Kearins MC, Conners K, Condon B, Maloney PC, Guggino WB, Hunt JF, Emtage S (2005) Impact of the DeltaF508 mutation in first nucleotide-binding domain of human cystic fibrosis transmembrane conductance regulator on domain folding and structure. J Biol Chem 280:1346–1353
Locher KP (2009) Structure and mechanism of ATP-binding cassette transporters. Philos Trans R Soc Lond B Biol Sci 364:239–245
Logan J, Hiestand D, Daram P, Huang Z, Muccio DD, Hartman J, Haley B, Cook WJ, Sorscher EJ (1994) Cystic fibrosis transmembrane conductance regulator mutations that disrupt nucleotide binding. J Clin Invest 94:228–236
Lu NT, Pedersen PL (2000) Cystic fibrosis transmembrane conductance regulator: the purified NBF1+R protein interacts with the purified NBF2 domain to form a stable NBF1+R/NBF2 complex while inducing a conformational change transmitted to the C-terminal region. Arch Biochem Biophys 375:7–20
Mense M, Vergani P, White DM, Altberg G, Nairn AC, Gadsby DC (2006) In vivo phosphorylation of CFTR promotes formation of a nucleotide-binding domain heterodimer. EMBO J 25:4728–4739
Mio K, Ogura T, Mio M, Shimizu H, Hwang T, Sato C, Sohma Y (2008) Three-dimensional reconstruction of human cystic fibrosis transmembrane conductance regulator chloride channel revealed an ellipsoidal structure with orifices beneath the putative transmembrane domain. J Biol Chem 283:30300–30310
Moran O, Galietta LJV, Zegarra-Moran O (2005) Binding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domains. Cell Mol Life Sci 62:446–460
Mornon J, Lehn P, Callebaut I (2009) Molecular models of the open and closed states of the whole human CFTR protein. Cell Mol Life Sci 66:3469–3486
Mylonas E, Svergun DI (2007) Accuracy of molecular mass determination of proteins in solution by small-angle X-ray scattering. J Appl Cryst 40:s245–s249
Neville DC, Rozanas CR, Tulk BM, Townsend RR, Verkman AS (1998) Expression and characterization of the NBD1-R domain region of CFTR: evidence for subunit-subunit interactions. Biochemistry 37:2401–2409
Qu BH, Strickland EH, Thomas PJ (1997) Localization and suppression of a kinetic defect in cystic fibrosis transmembrane conductance regulator folding. J Biol Chem 272:15739–15744
Rosenberg MF, Kamis AB, Aleksandrov LA, Ford RC, Riordan JR (2004) Purification and crystallization of the cystic fibrosis transmembrane conductance regulator (CFTR). J Biol Chem 279:39051–39057
Sharma S, Rose DR (1995) Cloning, overexpression, purification, and characterization of the carboxyl-terminal nucleotide binding domain of P-glycoprotein. J Biol Chem 270:14085–14093
Sreerama N, Woody RW (2004) Computation and analysis of protein circular dichroism spectra. Methods Enzymol 383:318–351
Svergun DI (1992) Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J Appl Cryst 25:495–503
Svergun DI (1999) Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys J 76:2879–2886
Svergun DI, Koch MH (2003) Small-angle scattering studies of biological macromolecules in solution. Rep Prog Phys 66:1735–1782
Thibodeau PH, Brautigam CA, Machius M, Thomas PJ (2005) Side chain and backbone contributions of Phe508 to CFTR folding. Nat Struct Mol Biol 12:10–16
Vergani P, Nairn AC, Gadsby DC (2003) On the mechanism of MgATP-dependent gating of CFTR Cl- channels. J Gen Physiol 121:17–36
Vergani P, Basso C, Mense M, Nairn AC, Gadsby DC (2005) Control of the CFTR channel’s gates. Biochem Soc Trans 33:1003–1007
Volkov V, Svergun D (2003) Uniqueness of ab initio shape determination in small-angle scattering. J Appl Cryst 36:860–864
Wriggers W (2010) Using Situs for the integration of multi-resolution structures. Biophys Rev 2:21–27
Wriggers W, Chacón P (2001) Using situs for the registration of protein structures with low-resolution bead models from X-ray solution scattering. J Appl Cryst 34:773–776
Zegarra-Moran O, Monteverde M, Galietta LJV, Moran O (2007) Functional analysis of mutations in the putative binding site for cystic fibrosis transmembrane conductance regulator potentiators. Interaction between activation and inhibition. J Biol Chem 282:9098–9104
Acknowledgments
We thank the ESRF for provision of synchrotron radiation facilities, and we would like to thank Petra Pernod for assistance in using beamline ID14-EH3. We thank Olga Zegarra, Gino Galietta, Paola Vergani, and Ilaria Zanardi for their comments. This project was supported by the Fondazione Ricerca Fibrosi Cistica (grant #2/2008), Mille bambini a Via Margutta–onlus, Blunotte and Lega Italiana FC–Associazione Toscana Onlus.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Galeno, L., Galfrè, E. & Moran, O. Small-angle X-ray scattering study of the ATP modulation of the structural features of the nucleotide binding domains of the CFTR in solution. Eur Biophys J 40, 811–824 (2011). https://doi.org/10.1007/s00249-011-0692-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-011-0692-5