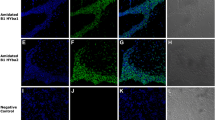
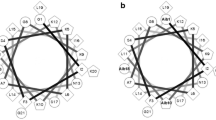
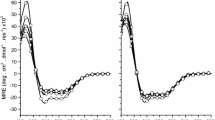
Abstract
Bombinins H are mildly cationic antimicrobial peptides isolated from the skin of the anuran genus Bombina, the fire-bellied toad. Some members of this peptide family coexist in skin secretions as diastereomers in which a single d-amino acid (alloisoleucine or leucine) is incorporated as a result of the post-translational modification of the respective gene-encoded l-amino acid. Here we report on the antimicrobial properties and membrane interactions of bombinins H2 and H4. The latter differs from H2 by the presence of a d-alloisoleucine at the second N-terminal position. Specifically, we have evaluated the antimicrobial activity of H2 and H4 against a large panel of reference and clinical isolates of Gram-negative and Gram-positive bacteria; performed membrane permeation assays on both intact cells and model membranes (lipid monolayers and liposomes) mimicking the composition of the plasma membrane of Gram-negative/positive bacteria; used biochemical tools, such as trypsin-encapsulated liposomes and capillary electrophoresis, to monitor the peptides’ ability to translocate through the membrane of liposomes mimicking Escherichia coli inner membrane. The results revealed interesting relationships between the presence of a single d-amino acid in the sequence of an antimicrobial peptide and its target microbial cell selectivity/membrane-perturbing activity.





Similar content being viewed by others
Abbreviations
- AMP:
-
Antimicrobial peptide
- CD:
-
Circular dichroism
- CFU:
-
Colony-forming units
- CL:
-
Cardiolipin
- CZE:
-
Capillary zone electrophoresis
- FITC-D4/D20/D70:
-
Fluorescein isothiocyanate-dextrans of 4, 20, and 70 kDa average molecular mass, respectively
- LPS:
-
Lipopolysaccharide
- MHB:
-
Mueller-Hinton broth
- MIC:
-
Minimum inhibitory concentration
- PBS:
-
Phosphate-buffered saline
- PE:
-
l-α-phosphatidylethanolamine
- PG:
-
l-α-phosphatidyl-dl-glycerol
- SBTI:
-
Soybean trypsin inhibitor
- SDS:
-
Sodium-dodecyl sulphate
- SPB:
-
Sodium phosphate buffer, pH 7.4 with 0.1 mM EDTA
References
Allen TM (1981) A study of phospholipid interactions between high-density lipoproteins and small unilamellar vesicles. Biochim Biophys Acta 640:385–397
Bechinger B, Lohner K (2006) Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim Biophys Acta 1758:1529–1539
Beisswenger C, Bals R (2005) Functions of antimicrobial peptides in host defense and immunity. Curr Protein Pept Sci 6:255–264
Boman HG (2000) Innate immunity and the normal microflora. Immunol Rev 173:5–16
Boman HG (2003) Antibacterial peptides: basic facts and emerging concepts. J Intern Med 254:197–215
Bozzi A, Mangoni ML, Rinaldi AC, Mignogna G, Aschi M (2008) Folding propensity and biological activity of peptides: the effect of a single stereochemical isomerization on the conformational properties of bombinins in aqueous solution. Biopolymers 89:769–778
Brockman H (1999) Lipid monolayers: why use half a membrane to characterize protein-membrane interactions? Curr Opin Struct Biol 9:438–443
Buczek O, Yoshikami D, Bulaj G, Jimenez EC, Olivera BM (2005) Post-translational amino acid isomerization: a functionally important D-amino acid in an excitatory peptide. J Biol Chem 280:4247–4253
CLSI (2006) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard, 7th edn. CLSI document M7–A7, vol. 26, no.2. Clinical and Laboratory Standards Institute, Wayne
Dempsey CE, Hawrani A, Howe RA, Walsh TR (2010) Amphipathic antimicrobial peptides—from biophysics to therapeutics? Protein Pept Lett 17:1334–1344
Den Hertog AL, Wong Fong Sang HW, Kraayenhof R, Bolscher JG, Van’t Hof W, Veerman EC, Nieuw Amerongen AV (2004) Interactions of histatin 5 and histatin 5-derived peptides with liposome membranes: surface effects, translocation and permeabilization. Biochem J 379:665–672
Easton DM, Nijnik A, Mayer ML, Hancock RE (2009) Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol 27:582–590
Epand RM, Epand RF (2009) Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim Biophys Acta 1788:289–294
Erspamer V, Melchiorri P, Falconieri-Erspamer G, Negri L, Corsi R, Severini C, Barra D, Simmaco M, Kreil G (1989) Deltorphins: a family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites. Proc Natl Acad Sci USA 86:5188–5192
Ganz T (2003) Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3:710–720
Gibson BW, Tang DZ, Mandrell R, Kelly M, Spindel ER (1991) Bombinin-like peptides with antimicrobial activity from skin secretions of the Asian toad, Bombina orientalis. J Biol Chem 266:23103–23111
Giuliani A, Rinaldi AC (2010) Antimicrobial peptides. Methods and protocols. Methods in molecular biology, vol 618. Humana Press, New York
Hancock RE, Diamond G (2000) The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol 8:402–410
Jilek A, Mollay C, Tippelt C, Grassi J, Mignogna G, Mullegger J, Sander V, Fehrer C, Barra D, Kreil G (2005) Biosynthesis of a D-amino acid in peptide linkage by an enzyme from frog skin secretions. Proc Natl Acad Sci USA 102:4235–4239
Kamatani Y, Minakata H, Kenny PT, Iwashita T, Watanabe K, Funase K, Sun XP, Yongsiri A, Kim KH, Novales-Li P et al (1989) Achatin-I, an endogenous neuroexcitatory tetrapeptide from Achatina fulica Ferussac containing a D-amino acid residue. Biochem Biophys Res Commun 160:1015–1020
Kreil G (1994) Conversion of L- to D-amino acids: a posttranslational reaction. Science 266:996–997
Kreil G (1997) D-amino acids in animal peptides. Annu Rev Biochem 66:337–345
Kuwada M, Teramoto T, Kumagaye KY, Nakajima K, Watanabe T, Kawai T, Kawakami Y, Niidome T, Sawada K, Nishizawa Y et al (1994) Omega-agatoxin-TK containing d-serine at position 46, but not synthetic omega-[L-Ser46]agatoxin-TK, exerts blockade of P-type calcium channels in cerebellar Purkinje neurons. Mol Pharmacol 46:587–593
Li XZ, Poole K, Nikaido H (2003) Contributions of MexAB-OprM and an EmrE homolog to intrinsic resistance of Pseudomonas aeruginosa to aminoglycosides and dyes. Antimicrob Agents Chemother 47:27–33
MacDonald RC, MacDonald RI, Menco BP, Takeshita K, Subbarao NK, Hu LR (1991) Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta 1061:297–303
Maget-Dana R (1999) The monolayer technique: a potent tool for studying the interfacial properties of antimicrobial and membrane-lytic peptides and their interactions with lipid membranes. Biochim Biophys Acta 1462:109–140
Maisetta G, Batoni G, Esin S, Florio W, Bottai D, Favilli F, Campa M (2006) In vitro bactericidal activity of human beta-defensin 3 against multidrug-resistant nosocomial strains. Antimicrob Agents Chemother 50:806–809
Mangoni ML, Grovale N, Giorgi A, Mignogna G, Simmaco M, Barra D (2000) Structure-function relationships in bombinins H, antimicrobial peptides from Bombina skin secretions. Peptides 21:1673–1679
Mangoni ML, Papo N, Saugar JM, Barra D, Shai Y, Simmaco M, Rivas L (2006) Effect of natural L- to D-amino acid conversion on the organization, membrane binding, and biological function of the antimicrobial peptides bombinins H. Biochemistry 45:4266–4276
Mangoni ML, Marcellini L, Simmaco M (2007) Biological characterization and modes of action of temporins and bombinins H, multiple forms of short and mildly cationic anti-microbial peptides from amphibian skin. J Pept Sci 13:603–613
Mangoni ML, Maisetta G, Di Luca M, Gaddi LM, Esin S, Florio W, Brancatisano FL, Barra D, Campa M, Batoni G (2008) Comparative analysis of the bactericidal activities of amphibian peptide analogues against multidrug-resistant nosocomial bacterial strains. Antimicrob Agents Chemother 52:85–91
Marcellini L, Borro M, Gentile G, Rinaldi AC, Stella L, Aimola P, Barra D, Mangoni ML (2009) Esculentin-1b(1–18)–a membrane-active antimicrobial peptide that synergizes with antibiotics and modifies the expression level of a limited number of proteins in Escherichia coli. FEBS J 276:5647–5664
Montecucchi PC, de Castiglione R, Piani S, Gozzini L, Erspamer V (1981) Amino acid composition and sequence of dermorphin, a novel opiate-like peptide from the skin of Phyllomedusa sauvagei. Int J Pept Protein Res 17:275–283
Mor A, Amiche M, Nicolas P (1992) Enter a new post-translational modification: D-amino acids in gene-encoded peptides. Trends Biochem Sci 17:481–485
Rinaldi AC (2002) Antimicrobial peptides from amphibian skin: an expanding scenario. Curr Opin Chem Biol 6:799–804
Rinaldi AC, Di Giulio A, Liberi M, Gualtieri G, Oratore A, Bozzi A, Schininà ME, Simmaco M (2001) Effects of temporins on molecular dynamics and membrane permeabilization in lipid vesicles. J Pept Res 58:213–220
Rinaldi AC, Mangoni ML, Rufo A, Luzi C, Barra D, Zhao H, Kinnunen PK, Bozzi A, Di Giulio A, Simmaco M (2002) Temporin L: antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem J 368:91–100
Selsted ME, Ouellette AJ (2005) Mammalian defensins in the antimicrobial immune response. Nat Immunol 6:551–557
Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta 1462:55–70
Simmaco M, Barra D, Chiarini F, Noviello L, Melchiorri P, Kreil G, Richter K (1991) A family of bombinin-related peptides from the skin of Bombina variegata. Eur J Biochem 199:217–222
Simmaco M, Mignogna G, Barra D (1998) Antimicrobial peptides from amphibian skin: what do they tell us? Biopolymers 47:435–450
Simmaco M, Kreil G, Barra D (2009) Bombinins, antimicrobial peptides from Bombina species. Biochim Biophys Acta 1788:1551–1555
Stewart JC (1980) Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem 104:10–14
Szoka F Jr, Papahadjopoulos D (1978) Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci USA 75:4194–4198
Torres AM, Menz I, Alewood PF, Bansal P, Lahnstein J, Gallagher CH, Kuchel PW (2002) D-amino acid residue in the C-type natriuretic peptide from the venom of the mammal, Ornithorhynchus anatinus, the Australian platypus. FEBS Lett 524:172–176
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Zhao H, Kinnunen PK (2002) Binding of the antimicrobial peptide temporin L to liposomes assessed by Trp fluorescence. J Biol Chem 277:25170–25177
Acknowledgments
This work was supported by grants from Università di Roma La Sapienza, MIUR (Italian Ministry of Education, University and Research; PRIN 2008), and Istituto di Biologia e Patologia Molecolari of the Italian National Research Council (CNR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Membrane-active peptides: 455th WE-Heraeus-Seminar and AMP 2010 Workshop.
Rights and permissions
About this article
Cite this article
Coccia, C., Rinaldi, A.C., Luca, V. et al. Membrane interaction and antibacterial properties of two mildly cationic peptide diastereomers, bombinins H2 and H4, isolated from Bombina skin. Eur Biophys J 40, 577–588 (2011). https://doi.org/10.1007/s00249-011-0681-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-011-0681-8