Abstract
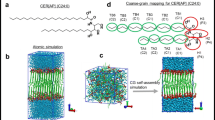
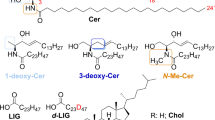
The lipid matrix in stratum corneum (SC) plays a key role in the barrier function of the mammalian skin. The major lipids are ceramides (CER), cholesterol (CHOL) and free fatty acids (FFA). Especially the unique-structured ω-acylceramide CER[EOS] is regarded to be essential for skin barrier properties by inducing the formation of a long-periodicity phase of 130 Å (LPP). In the present study, the arrangement of CER[EOS], either mixed with CER[AP] and CHOL or with CER[AP], CHOL and palmitic acid (PA), inside a SC lipid model membrane has been studied for the first time by neutron diffraction. For a mixed CER[EOS]/CER[AP]/CHOL membrane in a partly dehydrated state, the internal membrane nanostructure, i.e. the neutron scattering length density profile in the direction normal to the surface, was obtained by Fourier synthesis from the experimental diffraction patterns. The membrane repeat distance is equal to that of the formerly used SC lipid model system composed of CER[AP]/CHOL/PA/ChS. By comparing both the neutron scattering length density profiles, a possible arrangement of synthetic long-chain CER[EOS] molecules inside a SC lipid model matrix is suggested. The analysis of the internal membrane nanostructure implies that one CER[EOS] molecule penetrates from one membrane layer into an adjacent layer. A 130 Å periodicity phase could not be observed under experimental conditions, either in CER/CHOL mixtures or in CER/CHOL/FFA mixture. CER[EOS] can be arranged inside a phase with a repeat unit of 45.2 Å which is predominately formed by short-chain CER[AP] with distinct polarity.




Similar content being viewed by others
Abbreviations
- CER[AP] :
-
N-(α-hydroxyoctadecanoyl)-phytosphingosine
- CER[EOS] :
-
30-Linoyloxy-triacontanoic acid-[(2S,3R)-1,3-dihydroxyocta-dec-4-en-zyl]-amide
- PA :
-
Palmitic acid
- CHOL :
-
Cholesterol
- ChS :
-
Cholesterol sulphate
References
Al-Amoudi A, Dubochet J, Norlén L (2005) Nanostructure of the epidermal extracellular space as observed by cryo-electron microscopi of vitreous sections of human skin. J Invest Dermatol 124:764–777
Ali MR, Cheng KH, Huang J (2006) Ceramide drives cholesterol out of the ordered lipid bilayer phase into the crystal phase in 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine/cholesterol/ceramide ternary mixtures. Biochemistry 45:12629–12638
Bouwstra JA, Gooris GS, Van der Spek JA, Bras WW (1991) Structural investigations of human stratum corneum by small angle x-ray scattering. J Invest Dermatol 97:1005–1012
Bouwstra JA, Gooris GS, Cheng K, Weerheim A, Bras W, Ponec M (1996) Phase behaviour of isolated skin lipids. J Lipid Res 37:999–1011
Bouwstra JA, Gooris GS, Weerheim AM, Ijzerman AP, Ponec M (1998) Role of ceramide 1 in the molecular organization of the stratum corneum lipids. J Lipid Res 39:186–196
Bouwstra JA, Dubbelaar FE, Gooris GS, Ponec M (2000) The lipid organisation in the skin barrier. Acta Derm Venereol Suppl 208:23–30
Bouwstra JA, Gooris GS, Dubbelaar FER, Ponec M (2001) Phase behaviour of lipid mixtures based on human ceramides. J Lipid Res 42:1759–1770
Bouwstra JA, Gooris GS, Dubbelaar FER, Ponec M (2002) Phase behavior of stratum corneum lipid mixtures based on human ceramides: the role of natural and synthetic ceramide 1. J Invest Dermatol 118:606–617
Bouwstra J, Gooris G, Charalambopoulou G, Steriotis T, Hauss T (2004) Skin lipid organisation. HMI Experimental Report. BIO-01-1601. Source: http://www.hmi.de/bensc/reports/2004
Brzustowicz MR, Cherezov V, Caffrey M, Stillwell W, Wassall SR (2002) Molecular organization of cholesterol in polyunsaturated membranes: microdomain formation. Biophys J 82:285–298
Charalambopoulou GC, Steriotis TA, Hauss T, Stubos AK, Kanellopoulos NK (2004) Structural alterations of fully hydrated human stratum corneum. Phys Rev B Condens Matter 350(1–3) (Suppl 1):E603–E606
Downing DT, Stewart ME, Wertz PW, Colton SW, Abraham W, Strauss JS (1987) Skin lipids: an update. J Invest Dermatol 88:2s–6s
Elias PM (1981) Epidermal lipids, membranes, and keratinisation. Int J Dermatol 20:1–19
Franks NP, Lieb WR (1979) The structure of lipid bilayers and the effects of general anaesthetics. J Mol Biol 133:469–500
Garson JC, Doucet J, Leveque JL, Tsoucaris G (1991) Oriented strcuture in human stratum corneum revealed by X-ray diffraction. J Invest Dermatol 96:43–49
Gordeliy VI, Kiselev MA (1995) Definition of lipid membrane structural parameters from neutronographic experiments with the help of the strip function model. Biophys J 69:1424–1428
Grubauer G, Feingold KR, Harris RM, Elias PM (1989) Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res 30:89–96
Hauss T, Dante S, Dencher NA, Haines TH (2002) Squalane is in the midplane of the lipid bilayer: implications for its function as a proton permeability barrier. Biochim Biophys Acta 1556:149–54
Huang J, Buboltz JT, Feigenson GW (1999) Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochi Biophys Acta 1417:89–100
Jager MW, Gooris GS, Dolbnya IP, Bras W, Ponec M, Bouwstra JA (2003) The phase behaviour of skin lipid mixtures based on synthetic ceramides. Chem Phys Lipids 124:123–134
Jager MW, Gooris GS, Ponec M, Bouwstra JA (2004a) Acylceramide head group architecture affects lipid organization in synthetic ceramide mixtures. J Invest Dermatol 123:911–916
Jager MW, Gooris GS, Dolbnya IP, Ponec M, Bouwstra JA (2004b) Modelling the stratum corneum lipid organization with synthetic lipid mixtures: the importance of synthetic ceramide compositions. Biochim Biophys Acta 1684:132–140
Jager MW, Gooris GS, Ponec M, Bouwstra JA (2005) Lipid mixtures prepared with well-defined synthetic ceramides closely mimic the unique stratum corneum lipid phase behavior. J Lipid Res 46:2649–2656
Jager MW, Groenink W, Bielsa R, Anderson E, Angelova N, Ponec M, Bouwstra JA (2006) A novel in vitro percutaeous penetration model: evaluation of barrier properties with p-aminobenzoic acid and two of its derivatives. Pharm Res 23:951–960
Katsaras J, Yang D, Epand RM (1992) Fatty-acid chain tilt angles and directions in dipalmitoyl phosphatidylcholine bilayers. Biophys J 63:1170–1175
Kessner D, Ruettinger A, Kiselev MA, Wartewig S, Neubert RHH (2008a) Properties of ceramides and their impact on the stratum corneum structure a review, part ii: stratum corneum lipid model systems. Skin Pharmacol Physiol 21:58–74
Kessner D, Kiselev MA, Hauß T, Dante S, Wartewig S, Neubert RHH (2008b) Localisation of partially deuterated cholesterol in quaternary SC lipid model membranes. A neutron diffraction study. Eur Biophy J Biophy Lett (published online)
King GI, White SH (1986) Determining bilayer hydrocarbon thickness from neutron diffraction measurements using strip-function models. Biophys J 49:1047–1054
Kiselev MA, Ryabova NYu, Balagurov AM, Dante S, Hauss Th, Zbytovska J, Wartewig S, Neubert RHH (2005) New insights into structure and hydration of SC lipid model membranes by neutron diffraction. Eur Biophys J 34:1030–1040
Madison KC, Swartzendruber DC, Wertz PW, Downing DT (1987) Presence of intact intercellular lamellae in the upper layers of the stratum corneum. J Invest Dermatol 88:714–718
McIntosh TJ, Stewart ME, Downing DT (1996) X-ray diffraction analysis of isolated skin lipids: reconstruction of intercellular lipid domains. Biochemistry 35:3649–3653
McIntosh TJ (2003) Organization of skin stratum corneum extracellular lamellae: diffraction evidence for asymmetric distribution of cholesterol. Biophys J 85:1675–1681
Nagle JF, Tristram-Nagle S (2000) Structure of lipid bilayers. Biochim Biophys Acta 1469:159–195
Norlén L, Plasencia Gil I, Simonsen A, Descouts P (2007) Human stratum corneum lipid organization as observed by atomic force microscopy on Langmuir–Blodgett films. J Struct Biol 158:386–400
Ohta N, Ban S, Tanaka H, Nakata S, Hatta I (2003) Swelling of the intercellular lipid lamellar structure with short repeat in hairless mouse stratum corneum as studied by X-ray diffraction. Chem Phys Lipids 123:1–8
Pascher I (1976) Molecular arrangement in sphingolipids. Conformation and hydrogen bonding of ceramide and their implication on membrane stability and permeability. Biochim Biophys Acta 455:433–451
Pfeiffer S, Vielhaber G, Vietzke JP, Wittern KP, Hintze U, Wepf R (2000) High-Pressure freezing provides new information on human epidermis: simultaneous protein antigen and lamellar lipid structure preservation. Study on human epidermis by cryoimmobilization. J Invest Dermatol 114:1030–1038
Ponec M, Weerheim A, Lankhorst P, Wertz P (2003) New acylceramide in native and reconstructed epidermis. J Invest Dermatol 120:581–588
Raith K, Farwanah H, Wartewig S, Neubert RHH (2004) Progress in the analysis of stratum corneum ceramides. Eur J Lipid Sci Technol 106:004–008
Raudenkolb S, Wartewig S, Brezesinski G, Funari SS, Neubert RH (2005) Hydration properties of N-(alpha-hydroxyacyl)-sphingosine: X-ray powder diffraction and FT-Raman spectroscopic studies. Chem Phys Lipids 136:13–22
Rerek ME, Chen H, Markovic B, Van Wyck D, Garidel P, Mendelsohn R, Moore D (2001) Phytosphingosine and spingosine ceramide headgroup hydrogen bonding: structural insights through thermotropic hydrogen/deuterium exchange. J Phys Chem 105:9355–9362
Robson KJ, Stewart ME, Michelsen S, Lazlo ND, Downing DT (1994) 6-Hydroxy-4-sphin-genine in human epidermal ceramides. J Lipid Res 35:2060–2068
Ruettinger A, Kiselev M, Hauss T, Dante S, Balagurov A, Neubert R (2008a) Fatty acid interdigitation in stratum corneum model membranes: a neutron diffraction study. Eur Biophys J (published online)
Ruettinger A, Kessner D, Kiselev MA, Hauss T, Dante S, Balagurov AM, Neubert RHH (2008b) Basic nanostructure of CER[EOS]/CER[AP]/CHOL/FFA multilamellar membrane. Neutron diffraction study. Biochim Biophys Acta (submitted 2008)
Swartzendruber DC, Wertz PW, Kitko DJ, Madison KC, Downing DT (1989) Molecular models of intercellular lipid lamellae in mammalian stratum corneum. J Invest Dermatol 92:251–257
Seul M, Sammon J (1990) Preparation of surfactant multilayer films on solid substrates by deposition from organic solution. Thin Solid Films 185:287–305
Steriotis T, Charalambopoulou G, Hauss T (2002) Structural analysis of hydrated stratum corneum HMI Experimental Report. BIO-01-1231. Source: http://www.hmi.de/bensc/reports/2002
Stewart ME, Downing DT (1999) A new 6-hydroxy-4-sphingenine-containing ceramide in human skin. J Lipid Res 4:1434–1439
Wertz PW, Miethke MC, Long SA, Strauss JS, Downing DT (1985) The composition of the ceramides from human stratum corneum and from comedones. J Invest Dermatol 84:410–412
Wertz PW, van den Bergh B (1998) The physical, chemical and functional properties of lipids in the skin and other biological barriers. Chem Phys Lipids 91:85–96
White S, Mirejosray D, King G (1988) Strcuture of lamellar domains and corneocytes envelopes in murine stratum corneum. Biochemistry 27:3725–3732
Wiener MC, White SC (1991) Fluid bilayer structure determination by the combined use of X-ray and neutron diffraction. Biophys J 59:162–173
Worcester DL (1976) Neutron diffraction studies of biological membranes and membrane components. Brookhaven Symp Biol 27:III37–II57
Acknowledgments
The authors are grateful to Professor Dr A. B. Balagurov for the calculations done for cholesterol crystal. Financial assistance of Hahn–Meitner Institute (Berlin, Germany) is gratefully acknowledged. D. Kessner would like to thank the Graduiertenförderung des Landes Sachsen–Anhalt for funding. The authors would like to thank Evonik Goldschmidt GmbH (Essen, Germany) for the gift of CER[EOS] and CER[AP].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kessner, D., Kiselev, M., Dante, S. et al. Arrangement of ceramide [EOS] in a stratum corneum lipid model matrix: new aspects revealed by neutron diffraction studies. Eur Biophys J 37, 989–999 (2008). https://doi.org/10.1007/s00249-008-0328-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-008-0328-6