Abstract
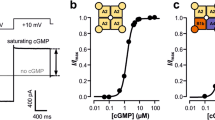
Three constructs are used for the analysis of biophysical properties of CNGA1 channels: the WT CNGA1 channel, a CNGA1 channel where all endogenous cysteines were removed (CNGA1cys-free) and a construct composed of two CNGA1 subunits connected by a small linker (CNGA1tandem). So far, it has been assumed, but not proven, that the molecular structure of these ionic channels is almost identical. The I/V relations, ionic selectivity to alkali monovalent cations, blockage by tetracaine and TMA+ were not significantly different. The cGMP dose response and blockage by TEA+ and Cd2+ were instead significantly different in CNGA1 and CNGA1cys-free channels, but not in CNGA1 and CNGA1tandem channels. Cd2+ blocked irreversibly the mutant channel A406C in the absence of cGMP. By contrast, Cd2+ did not block the mutant channel A406C in the CNGA1cys-free background (A406Ccys-free), but an irreversible and almost complete blockage was observed in the presence of the cross-linker M–4–M. Results obtained with different MTS cross-linkers and reagents suggest that the 3D structure of the CNGA1cys-free differs from that of the CNGA1 channel and that the distance between homologous residues at position 406 in CNGA1cys-free is longer than in the WT CNGA1 by several Angstroms.





Similar content being viewed by others
Abbreviations
- CNG:
-
Cyclic nucleotide-gated
- CNBD:
-
Cyclic nucleotide-binding domain
- CSM:
-
Cysteine scanning mutagenesis
- MTS:
-
Methanethiosulfonate
- MTSET:
-
2-(Trimethylammonium)ethyl] methanethiosulfonate bromide
- MTSPT:
-
3-(Trimethylammonium)propyl methanethiosulfonate bromide
- MTS-PtrEA:
-
3-(Triethylammonium)propyl methanthiosulfonate bromide
- M–2–M:
-
1,2-Ethanediyl bismethanethiosulfonate
- M–4–M:
-
1,4-Butanediyl bismethanethiosulfonate
- M–6–M:
-
1,6-Hexanediyl bismethanethiosulfonate
- M–8–M:
-
3,6-Dioxaoctane-1,8-diyl bismethanethiosulfonate
- M–11–M:
-
3,6,9-Trioxaundecane-1,11-diyl bismethanethiosulfonate
References
Akabas MH, Stauffer DA, Xu M, Karlin A (1992) Acetylcoline receptor channel structure probed in cysteine-substitution mutants. Science 258:307–310
Becchetti A, Gamel K (1999) The properties of cysteine mutants in the pore region of cyclic-nucleotide-gated channels. Pflugers Arch 438:587–596
Becchetti A, Gamel K, Torre V (1999) Cyclic nucleotide-gated channels pore topology studied through the accessibility of reporter cysteins. J Gen Physiol 114:377–392
Biel M, Zong X, Ludwing FA, Sautter A, Hofmann F (1999) Structure and function of cyclic nucleotide-gated channels. Rev Physiol Biochem Pharmacol 135:151–171
Bradley J, Frings S, Yau KW, Reed R (2001) Nomenclature for ion channel subunits. Science 294:2095–2096
Brown RL, Snow SD, Haley TL (1998) Movement of gating machinery during activation of rod cyclic nucleotide-gated channels. Biophys J 75:825–833
Bucossi G, Eismann E, Sesti F, Nizzari M, Seri M, Kaupp UB, Torre V (1996) Time-dependent current decline in cyclic GMP-gated bovine channels caused by point mutations in the pore region expressed in Xenopus oocytes. J Physiol (Lond) 493:409–418
Bucossi G, Nizzari M, Torre V (1997) Single-channel properties of ionic channels gated by cyclic nucleotides. Biophys J 72:1165–1181
Colamartino G, Menini A, Torre V (1991) Blockage and permeation of divalent cations through the cyclic GMP-activated channel from tiger salamander retinal rods. J Physiol 440:189–206
Contreras JE, Holmgren M (2006) Access of quaternary ammonium blockers to the internal pore of cyclic nucleotide-gated channels: implications for the location of the gate. J Gen Physiol 127:481–494
Craven KB, Zagotta WN (2006) CNG and HCN channels: two peas, one pod. Annu Rev Physiol 68:375–401
Eismann E, Muller F, Heinemann SH, Kaupp UB (1994) A single negative charge within the pore region of a cGMP-gated channel controls rectification, Ca2+ blockage, and ionic selectivity. Proc Natl Acad Sci USA 91:1109–1113
Fesenko EE, Kolesnikov SS, Lyubarsky AL (1985) Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 313:310–313
Flynn GE, Zagotta WN (2003) A cysteine scan of the inner vestibule of cyclic nucleotide gated channels reveals architecture and rearrangement of the pore. J Gen Physiol 121:563–582
Fodor AA, Black KD, Zagotta WN (1997a) Tetracaine reports a conformational change in the pore of cyclic nucleotide-gated channels. J Gen Physiol 110:591–600
Fodor AA, Gordon SE, Zagotta WN (1997b) Mechanism of tetracaine block of cyclic nucleotide-gated channels. J Gen Physiol 109:3–14
Glusker JP (1991) Structural aspects of metal liganding to functional groups in proteins. Adv Protein Chem 42:1–76
Gordon SE, Varnum MD, Zagotta WN (1997) Direct interaction between amino- and carboxyl-terminal domains of cyclic nucleotide-gated channels. Neuron 19:431–441
Gordon SE, Zagotta WN (1995) Subunit interactions in coordination of Ni2+ in cyclic nucleotide-gated channels. Proc Natl Acad Sci USA 92:10222–10226
Hastrup H, Karlin A, Javitch JA (2001) Symmetrical dimer of the human dopamine transporter revealed by cross-linking Cys-306 at the extracellular end of the sixth transmembrane segment. Proc Natl Acad Sci USA 98:10055–10060
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Jan LY, Jan YN (1990) A superfamily of ion channels. Nature 345:672
Karlin A, Akabas MH (1998) Substituted-cysteine accessibility method. In: Conn PM (Ed) Methods in enzimology. Academic Press, San Diego pp. 123–145
Kato H, Tanaka T, Nishioka T, Kimura A, Oda J (1988) Role of cysteine residues in glutathione synthetase from Escherichia coli B. Chemical modification and oligonucleotide site-directed mutagenesis. J Biol Chem 263:11646–11651
Kaupp UB, Niidome T, Tanabe T, Terada S, W.Bönigk, W.Stühmer, Cook NJ, Kangawa K, Matsuo H, Hirose T, Miyata T, Numa S (1989) Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature 342:762–766
Kaupp UB, Seifert R (2002) Cyclic nucleotide gated channels. Physiol Rev 82:769–824
Kohler K, Forster IC, Stange G, Biber J, Murer H (2003) Essential cysteine residues of the type IIa Na+/Pi cotransporter. Pflugers Arch 446:203–210
Körschen HG, Illing M, Seifert R, Sesti F, Willams A, Gotzes S, Colville C,Müller F, Dosé A, Godde M (1995) A 240 kDa protein represents the complete beta subunit of the cyclic nucleotide-gated channel from rod photoreceptor. Neuron 15:627–636
Liu J, Siegelbaum SA (2000) Change of pore helix conformational state upon opening of cyclic nucleotide gated channels. Neuron 28:899–909
Loo TW, Clarke DM (2001) Determining the dimensions of the drug-binding domain of human P-glycoprotein using thiol cross-linking compounds as molecular rulers. J Biol Chem 276:36877–36880
Matulef K, Flynn GE, Zagotta WN (1999) Molecular rearrangements in the ligand-binding domain of cyclic nucleotide-gated channels. Neuron 24:443–452
Matulef K, Zagotta W (2002) Multimerization of the ligand binding domains of cyclic nucleotide-gated channels. Neuron 36:93–103
Matulef K, Zagotta WN (2003) Cyclic nucleotide-gated ion channels. Annu Rev Cell Dev Biol 19:23–44
Menini A (1990) Currents carried by monovalent cations through cyclic GMP-activated channels in excised patches from salamander rods. J Physiol 424:167–185
Molday RS, Molday LL, Dose A, I.Clark-Lewis, Illing M, Cook NJ, Eismann E, Kaupp UB (1991) The cGMP-gated channel of the rod photoreceptor cell characterization and orientation of the amino terminus. J Biol Chem 266:21917–21922
Nair AV, Mazzolini M, Codega P, Giorgetti A, Torre V (2006) Locking CNGA1 channels in the open and closed state. Biophys J 90:3599–3607
Nizzari M, Sesti F, Giraudo MT, Virginio C, Cattaneo A, Torre V (1993) Single-channel properties of cloned cGMP-activated channels from retinal rods. Proc R Soc Lond 254:69–74
Picco C, Menini A (1993) The permeability of the cGMP-activated channel to organic cations in retinal rods of the tiger salamander. J Physiol 460:741–758
Ren X, Nicoll DA, Philipson KD (2006) Helix packing of the cardiac Na+–Ca2+ exchanger: proximity of transmembrane segments 1, 2, and 6. J Biol Chem 281:22808–22814
Root MJ, MacKinnon R (1993) Identification of an external divalent binding site in the pore of a cGMP-activated channel. Neuron 11:459–466
Rosenbaum T, Gordon SE (2002) Dissecting intersubunit contacts in cyclic nucleotide-gated ion channels. Neuron 33:703–713
Rothberg B, Shin K, Phale P, Yellen G (2002) Voltage-controlled gating at the intracellular entrance to a hyperpolarization-activated cation channel. J Gen Physiol 119:83–91
Sesti F, Eismann E, Kaupp UB, Nizzari M, Torre V (1995) The multi-ion nature of the cGMP-gated channel from vertebrate rods. J Physiol (Lond) 487:17–36
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611
Snedecor GW, Cochran WG (1989) Statistical methods, 8th edn. Blackwell, Oxford, pp 98–99
Sullivan DA, Cohen JB (2000) Mapping the agonist binding site of the nicotinic acetylcholine receptor. Orientation requirements for activation by covalent agonist. J Biol Chem 275:12651–12660
Sun ZP, Akabas MH, Goulding EH, Karlin A, Siegelbaum SA (1996) Exposure of residues in the cyclic nucleotide-gated channel pore: P region structure and function in gating. Neuron 16:141–149
Taylor AM, Storm J, Soceneantu L, Linton KJ, Gabriel M, Martin C, Woodhouse J, Blott E, Higgins CF, Callaghan R (2001) Detailed characterization of cysteine-less P-glycoprotein reveals subtle pharmacological differences in function from wild-type protein. Br J Pharmacol 134:1609–1618
Varnum MD, Zagotta WN (1996) Subunit interactions in the activation of cyclic nucleotide-gated ion channels. Biophys J 70:2667–2679
Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB (2002) Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron 36:881–889
White D, Taverner CB, Leach PGL, Coville NJ (1993) Quantification of substituent and ligand size by the use of solid angles. J Comp Chem 14:1042–1049
Zagotta WN, Siegelbaum SA (1996) Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci 19:235–263
Zheng J, Trudeau MC, Zagotta WN (2002) Rod cyclic nucleotide gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron 36:891–896
Zhong H, Molday LL, Molday RS, Yau KW (2002) The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature 420:193–198
Acknowledgments
We are extremely thankful to William Zagotta who very generously supplied us with the clone of the CNGA1 and CNGA1cys-free channels and Claudio Anselmi for helpful discussions. This work was supported by a HFSP grant, a COFIN grant from the Italian Ministry, a grant from CIPE (GRAND FVG) and a FIRB grant from MIUR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mazzolini, M., Nair, A.V. & Torre, V. A comparison of electrophysiological properties of the CNGA1, CNGA1tandem and CNGA1cys-free Channels. Eur Biophys J 37, 947–959 (2008). https://doi.org/10.1007/s00249-008-0312-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-008-0312-1