Abstract
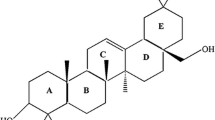
The red yeast Xanthophyllomyces dendrorhous is one of the microbiological production systems for natural carotenoids. High-performance liquid chromatography (HPLC) and electron paramagnetic resonance spectroscopy (EPR) experiments were performed on X. dendrorhous membranes in order to study the effect of incorporation rates of different type of carotenoids. In the case of fluid-phase membranes, it was found that polar carotenoids, such as astaxanthin and cis-astaxanthin, increased the EPR order parameter and decreased the motional freedom and phase-transition temperature. In contrast the non-polar carotenoids β-cryptoxanthin and β-carotene decreased the EPR order parameter and increased motional freedom and phase-transition temperature. A noteworthy coherence was observed between the polarities of the strains and the phase-transition temperatures.







Similar content being viewed by others
References
Andrewes AG, Phaff HJ, Starr MP (1976) Carotenoids of Phaffia rhodozyma, a red-pigmented fermenting yeast. Phytochemistry 15:1003–1007
Baltschun D, Beutner S, Biriviba K, Stenhorst F (1997) Singlet oxygen quenching abilities of carotenoids. Liebigs Ann/Recueil 1887–1893
Belagyi J, Pas M, Raspor P, Pesti M, Pali T (1999) Effect of hexavalent chromium on eukaryotic plasma membrane studied by EPR spectroscopy. Biochim Biophys Acta 1421:175–182
Deli J, Molnar P (2002) Paprika carotenoids: analysis, isolation, structure elucidation. Curr Org Chem 6:1197–1219
Farkas N, Pesti M, Belagyi J (2003) Effect of hexavalent chromium on the plasma membranes of sensitive and tolerant mutants of Schizosaccharomyces pombe. An EPR study. Biochim Biophys Acta 1611:217–222
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265
Gabrielska J, Gruszecki WI (1996) Zeaxanthin (dihydroxy-β-carotene) but not β-carotene rigidifies lipid membranes: a 1H-NMR study of carotenoids-egg phosphatidylcholine liposomes. Biochim Biophys Acta 1285:167–174
Gaffney BJ (1976) Practical consideration for calculation of order parameters for fatty acid or phospholipids spin labels in membranes. In: Berliner LJ (ed) Spin labeling: theory and applications, vol 1. Academic Press, New York, pp 567–571
Girard J, Falconnier B, Bricout J, Vladescu B (1994) β-Carotene producing mutants of Phaffia rhodozyma. Microbiol Biotechnol 41:183–191
Goldman SA, Bruno GV, Freed JH (1972) Estimating slow-motional rotational correlation times for nitroxides by electron spin resonance. J Phys Chem 76:1858–1869
Goto S, Kogure K, Abe K, Kimata Y, Kitahama K, Yamashita E, Terada H (2001), Efficient radical trapping at the surface and inside the phospholipids membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim Biophys Acta 1512:251–258
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press, Oxford
Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM (2006) Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr 46:185–196
Isralachvili J, Sjönsten J, Ericsson LEG, Mhrström M, Graslund A, Ehrenberg A (1974) Theoretical analysis of the molecular motion of spin labels in membranes. ESR spectra of labeled Bacillus subtilis membranes. Biochim Biophys Acta 339:164–172
Jezowska I, Wolak A, Gruszecki WI, Strzalka K (1994) Effect of β-carotene on structural and dynamic properties of model phosphatidylcholine membranes. II. A 31P-NMR and 13C-NMR study. Biochim Biophys Acta 1194:143–148
Johnson EA, Lewis MJ (1979) Astaxanthin formation by the yeast Phaffia rhodozyma. J Gen Microbiol 115:173–183
Jones RH, Molitoris BA (1984) A statistical method for determining the breakpoint of two lines. Anal Biochem 141:287–290
Lee J, Dawes I, Roe JH (1995) Adaptive response of Schizosaccharomyces pombe to hydrogen peroxide and menadion. Microbiology 141:3127–3132
Libkind D, Brizzio S, Broock M (2003) Rhodotorula mucilaginosa, a carotenoid producing yeast strain from a Patagonian high-altitude lake. Folia Microbiol 49:19–25
Lim BP, Nagao A, Terao J, Tanaka K, Suzuki T, Takama K (1992) Antioxidant activity of xanthophylls on peroxyl radical-mediated phospholipids peroxidation. Biochim Biophys Acta 1126:178–184
Marsh D (1981) Electron spin resonance: spin labels. In: Grell E (ed) Membrane spectroscopy, Springer, Berlin, pp 52–198
Mortensen A, Skibsted LH, Sampson J, Rice-Evans C, Everett SA (1997) Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett 418:91–97
Niewska AW, Draus J, Subczynski WK (2003) Is a fluid-mosaic model of biological membranes fully relevant? Studies on lipid organization in model and biological membranes. Cell Mol Biol Lett 8:147–159
Palagyi Zs, Nagy Á, Vágvölgyi Cs, Ferenczy L (1995) A new mutation protocol for obtaining mutants of the yeast Phaffia rhodozyma. Biotechnol Technol 9:401–402
Palagyi Zs, Linka TB, Papp T, Vágvölgyi Cs (2006) Isolation and characterization of Xanthophyllomyces dendrorhous mutants with altered carotenoid content. Acta Aliment Hung 35:223–228
Pocsi I, Prade RA, Penninclex MJ (2004) Glutathione, a metabolite in fungi. Adv Microb Physiol 49:1–76
Rengel D, Díez-Navajas A, Serna-Rico A, Veiga P, Muga A, Milicua JCG (2000) Exogenously incorporated ketocarotenoids in large unilamellar vesicles. Protective activity against peroxidation. Biochim Biophys Acta 1463:179–187
Schroeder WA, Johnson EA (1993) Antioxidant role of carotenoids in Phaffia rhodozyma. J Gen Microbiol 139:907–912
Schroeder WA, Johnson EA (1995) Single oxygen and peroxyl radicals regulate carotenoid biosynthesis in Phaffia rhodozyma. J Biol Chem 270:18374–18379
Sedmak JJ, Weerasinghe DK, Jolly SO (1990) Extraction and quantitation of astaxanthin from Phaffia rhodozyma. Biotechnol Technol 4:107–112
Strazalka K, Gruszecki WI (1994) Effect of β-carotene on structural and dynamic properties of model phosphatidylcholine membranes. I. An EPR spin label study. Biochim Biophys Acta 1194:138–142
Subczynski WK, Wisniewska A (2000) Physical properties of lipid bilayer membranes: relevance to membrane biological functions. Cell Mol Biol Lett 47:613–623
Subczynski WK, Markowska E, Gruszecki WI (1992) Effect of polar carotenoids on dimyristoylphosphatidylcholine membranes: a spin-label study. Biochim Biophys Acta 1105:97–108
Subczynski WK, Markowska E, Sielewiesiuk J (1993) Spin-label studies on phosphatidylcholine-polar carotenoids membranes: effect of alkyl-chain length and unsaturation. Biochim Biophys Acta 1150:173–181
Visser H, Ooyen AJJ, Verdoes JC (2003) Metabolic engineering of the astaxanthin-biosynthetic pathway of Xanthophyllomyces dendrorhous. FEMS Yeast Res 4:221–231
Wataru M (1991) Biological functions and activities of animal carotenoids. Pure Appl Chem 63:141–146
Wisniewska A, Subczynski WK (1998) Effect of polar carotenoids on the shape of the hydrophobic barrier of phospholipids bilayers. Biochim Biophys Acta 1368:235–246
Woodall AA, Britton G, Jackson MJ (1997) Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: relationship between carotenoid structure and protective ability. Biochim Biophys Acta 1336:575–586
Author information
Authors and Affiliations
Corresponding author
Additional information
Regional Biophysics Conference of the National Biophysical Societies of Austria, Croatia, Hungary, Italy, Serbia, and Slovenia.
Rights and permissions
About this article
Cite this article
Blasko, A., Belagyi, J., Dergez, T. et al. Effect of polar and non-polar carotenoids on Xanthophylomyces dendrorhous membranes by EPR. Eur Biophys J 37, 1097–1104 (2008). https://doi.org/10.1007/s00249-008-0289-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-008-0289-9