Abstract
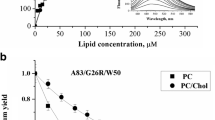
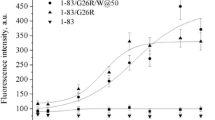
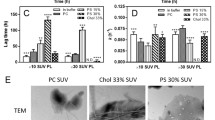
An amphipathic class A peptide, Ac-18A-NH2, has been employed in modeling the α-helical lipid-binding site of apolipoprotein A-I (apoA-I). To gain insight into the nature of protein–lipid interactions responsible for the ability of apoA-I to promote the efflux of intracellular cholesterol, the peptide disposition in model membranes composed of phosphatidylcholine (PC) and its mixture with cholesterol (Chol) has been characterized. By examining resonance energy transfer between the peptide Trp as a donor and anthrylvinyl-labeled PC as an acceptor it was found that Chol inclusion is conducive to shallower bilayer location of the Ac-18A-NH2 α-helix. The limits for the Trp distance from the membrane center were estimated to be 1.5–1.7 nm (PC) and 1.9–2.1 nm (PC:Chol), indicating that in the PC bilayer the Trp resides at the level of the glycerol backbone and carbonyl groups while the region of the phosphocholine moieties is preferable for Trp location in the PC:Chol bilayer. These findings suggest that Chol can modulate the interactions between apoA-I and membrane lipids via reducing the depth of α-helix bilayer penetration.


Similar content being viewed by others
Abbreviations
- apoA-I:
-
apolipoprotein A-I
- AV-PC:
-
anthrylvinyl-labeled phosphatidylcholine
- Chol:
-
cholesterol
- HDL:
-
high-density lipoproteins
- LUV:
-
large unilamellar vesicles
- PC:
-
phosphatidylcholine
- RET:
-
fluorescence resonance energy transfer
References
Albinsson B, Kubista M, Norden B, Thulstrup E (1989) Near-ultraviolet electronic transitions of the tryptophan fluorophore: linear dichroism, fluorescence anisotropy, and magnetic circular dichroism spectra of some indole derivatives. J Phys Chem 93:6646–6654
Anantharamaiah G, Jones J, Brouilette C, Schmidt C, Chung B, Hughes T, Brown A, Segrest J (1985) Studies of synthetic peptide analogs of the amphipathic helix: structure of complexes with dimyristoylphosphatidylcholine. J Biol Chem 260:10248–10255
Bartlett G (1959) Phosphorus assay in column chromatography. J Biol Chem 234:466–468
Braun P, Heijne G (1999) The aromatic residues Trp and Phe have different effects on the positioning of a transmembrane helix in the microsomal membrane. Biochemistry 38:9778–9782
Bulychev AA, Verchoturov VN, Gulaev BA (1988) Current methods of biophysical studies. Vyschayashkola, Moscow.
Clayton A, Sawyer W (1999) The structure and orientation of class A amphipathic peptides on a phospholipid bilayer surface. Eur Biophys J 28:133–141
Clayton A, Sawyer W (2000a) Site-specific tryptophan dynamics in class A amphipathic helical peptides at a phospholipid bilayer interface. Biophys J 79:1066–1073
Clayton A, Sawyer W (2000b) Oriented circular dichroism of a class A amphipathic helix in aligned phospholipid multilayers. Biochim Biophys Acta 1467:124–130
Dale R, Eisinger J, Blumberg W (1979) The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys J 26:161–194
Davenport L, Dale R, Bisby R, Cundall R (1985) Transverse location of the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene in model lipid bilayer membrane systems by resonance excitation energy transfer. Biochemistry 24: 4097–4108
Demel R, de Kruijff B (1976) The function of sterols in membranes. Biochim Biophys Acta 457:109–132
De Planque MR, Kruijtzer JA, Liskamp RM, Marsh D, Greathouse DV, Koeppe RE II, de Kruijff B, Killian JA (1999) Different membrane anchoring positions of tryptophan and lysine in synthetic transmembrane α-helical peptides. J Biol Chem 274:20839–20846
Duzgunes N, Shavnin S (1992) Membrane destabilization by N-terminal peptides of viral envelope proteins. J Membr Biol 128:71–80
Egashira M, Gorbenko G, Tanaka M, Saito H, Molotkovsky J, Nakano M, Handa T (2002) Cholesterol modulates interaction between an amphipathic class A peptide, Ac-18A-NH2, and phosphatidylcholine bilayers. Biochemistry 41:4165–4172
Epand R, Shai Y, Segrest J, Anantharamaiah G (1995) Mechanisms for the modulation of membrane bilayer properties by amphipathic helical peptides. Biopolymers 37:319–338
Fung BK, Stryer L (1978) Surface density determination in membranes by fluorescence energy transfer. Biochemistry 17:5241–5248
Ho C, Slater S, Stubbs C (1995) Hydration and order in lipid bilayers. Biochemistry 34:6188–6195
Hristova K, Wimley W, Mishra V, Anantharamaiah G, Segrest J, White S (1999) An amphipathic α-helix at a membrane interface: a structural study using a novel X-ray diffraction method. J Mol Biol 290:99–117
Ivkov VG, Berestovsky GN (1981) Dynamic structure of lipid bilayers. Nauka, Moscow
Johansson L, Molotkovsky J, Bergelson L (1990) Fluorescence properties of anthrylvinyl lipid probes. Chem Phys Lipids 53:185–189
Johnson WJ, Mahlberg FH, Rothblat GH, Phillips MC (1991) Cholesterol transport between cells and high-density lipoproteins. Biochim Biophys Acta 1085:273–298
Kirby E, Steiner R (1970) The influence of solvent and temperature upon the fluorescence of indole derivatives. J Phys Chem 74:4480–4490
Lakowicz JR (1999) Principles of fluorescent spectroscopy. Plenum Press, New York
Lecompte M, Bras A, Dousset N, Portas I, Salvayre R, Ayrault-Jarrier M (1998) Binding steps of apolipoprotein A-I with phospholipid monolayers: adsorption and penetration. Biochemistry 37:16165–16171
Levine Y (1972) Physical studies of membrane structure. Prog Biophys Mol Biol 24:1–74
Lu B, Morrow J, Weisgraber K (2000) Conformational reorganization of the four-helix bundle of human apolipoprotein E in binding to phospholipids. J Biol Chem 275:20775–20781
Lund-Katz S, Phillips M, Mishra V, Segrest J, Anantharamaiah G (1995) Microenvironments of basic amino acids in amphipathic α-helices bound to phospholipid: 13C NMR studies using selectively labeled peptides. Biochemistry 34:9219–9226
Maiorano N, Davidson S (2000) The orientation of helix 4 in apolipoprotein A-I-containing reconstituted high density lipoproteins. J Biol Chem 275:17374–17380
McLaughlin A, Cullis P, Hemminga M, Hoult D, Radda G, Ritchie G, Seeley P, Richards R (1975) Application of 31P NMR to model and biological membrane systems. FEBS Lett 57:213–218
Mishra V, Palgunachari M (1996) Interaction of model class A1, class A2 and class Y amphipathic helical peptides with membranes. Biochemistry 35:11210–11220
Mishra V, Palgunachari M, Segrest J, Anantharamaiah G (1994) Interactions of synthetic peptide analogs of the class A amphipathic helix with lipids. J Biol Chem 26:7185–7191
Mishra V, Palgunachari M, Lund-Katz S, Phillips M, Segrest J, Anantharamaiah G (1995) Effect of the arrangement of tandem repeating units of class A amphipathic α-helices on lipid interaction. J Biol Chem 270:1602–1611
Mishra V, Palgunachari M, Datta G, Phillips M, Lund-Katz S, Adeyeye S, Segrest J, Anantharamaiah G (1998) Studies of synthetic peptides of human apolipoprotein A-I containing tandem amphipathic α-helices. Biochemistry 37:10313–10324
Molotkovsky J, Dmitriev P, Nikulina L, Bergelson L (1979) Synthesis of new fluorescence labeled phosphatidylcholines. Bioorg Khim 5:588–594
Molotkovsky J, Dmitriev P, Molotkovskaya I, Bergelson L, Manevich E (1981) Synthesis of new fluorescent phospholipids and a study of their behavior in model membranes. Bioorg Khim 7:586–600
Monette M, Van Calsteren M, Lafleur M (1993) Effect of cholesterol on the polymorphism of dipalmitoylphosphatidylcholine/melittin complexes: an NMR study. Biochim Biophys Acta 1149:319–328
Nicol F, Nir S, Szoka F (1996) Effect of cholesterol and charge on pore formation in bilayer vesicles by a pH-sensitive peptide. Biophys J 71:3288–3301
Oram JF, Yokoyama S (1996) Apolipoprotein-mediated removal of cellular cholesterol and phospholipids. J Lipid Res 37:2473–2491
Palgunachari M, Mishra V, Lund-Katz S, Phillips M, Adeyeye S, Alluri S, Anantharamaiah G, Segrest J (1996) Only the two end helices of eight tandem amphipathic helical domains of human apoA-I have significant lipid affinity: implications for HDL assembly. Arteriorscler Thromb Vasc Biol 16:328–338
Persson S, Killian JA, Lindblom G (1998) Molecular ordering of interfacially localized analogs of ester- and ether-lipid bilayers studied by H-NMR. Biophys J 75:1365–1371
Polozov I, Polozova A, Tytler E, Anantharamaiah G, Segrest J, Wooley G, Epand R (1997) Role of lipids in the permeabilization of membranes by class L amphipathic helical peptides. Biochemistry 36:9237–9245
Pott T, Dufourc E (1995) Action melittin on the DPPC–cholesterol liquid-ordered phase: a solid state 2H- and 31P-NMR study. Biophys J 68:965–977
Saito H, Miyako Y, Handa T, Miyajima K (1997) Effect of cholesterol on apolipoprotein A-I binding to lipid bilayers and emulsions. J Lipid Res 38:287–294
Segrest J, de Loof H, Dohlman J, Brouilette C, Anantharamaiah G (1990) Amphipathic helix motif: classes and properties. Proteins Struct Funct Genet 8:103–117
Slotter JP, Oram JF, Bierman EL (1987) Binding of high density lipoproteins to cell receptors promotes translocation of cholesterol from intracellular membranes to the cell surface. J Biol Chem 262:12904–12907
Spuhler P, Anantharamaiah G, Segrest J, Seelig J (1994) Binding of apolipoprotein A-I model peptides to lipid bilayers. Measurement of binding isotherms and peptide–lipid headgroup interactions. J Biol Chem 269:23904–23910
Straume M, Litman B (1987) Influence of cholesterol on equilibrium and dynamic bilayer structure of unsaturated acyl chain phosphatidylcholine vesicles as determined from higher order analysis of fluorescence anisotropy decay. Biochemistry 26:5121–5126
Sviridov D, Pyle L, Fidge N (1996) Identification of a sequence of apolipoprotein A-I associated with the efflux of intracellular cholesterol to human serum and apolipoprotein A-I containing particles. Biochemistry 35:189–196
Tytler E, Segrest J, Epand RM, Nie S, Epand RF, Mishra V, Venkatachalapathi Y, Anantharamaiah G (1993) Reciprocal effects of apolipoprotein and lytic peptide analogs on membranes. Cross-sectional molecular shapes of amphipathic alpha helixes control membrane stability. J Biol Chem 268:22112–22118
Valeur B, Weber G (1977) Resolution of the fluorescence excitation spectrum of indole into 1La and 1Lb excitation bands. Photochem Photobiol 25:441–444
Venkatachalapathi Y, Phillips M, Epand RM, Epand RF, Tytler E, Segrest J, Anantharamaiah G (1993) Effect of end group blockage on the properties of a class A amphipathic helical peptide. Proteins Struct Funct Genet 15:349–359
Wiener M, White S (1992) Structure of a fluid dioleoylphosphatidylcholine bilayer determined by joint refinement of X-ray and neutron diffraction data. Biophys J 61:434–447
Wu P, Brand L (1992) Orientation factor in steady state and time-resolved resonance energy transfer measurements. Biochemistry 31:7939–7947
Yau W-M, Wimley W, Gawrisch K, White S (1998) The preference of tryptophan for membrane interface. Biochemistry 37:14713–14718
Yeagle P, Hutton W, Huang C, Martin R (1977) Phospholipid headgroup conformations: intermolecular interaction and cholesterol effects. Biochemistry 16:4344–4449
Yokoyama S (1998) Apolipoprotein-mediated cellular cholesterol efflux. Biochim Biophys Acta 1392:1–15
Acknowledgements
This work was supported in part by grants from Research Fellowships of the Japan Society for the Promotion of Science (RC 30026103 for G.G. and 12470488 for T.H.)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gorbenko, G., Handa, T., Saito, H. et al. Effect of cholesterol on bilayer location of the class A peptide Ac-18A-NH2 as revealed by fluorescence resonance energy transfer. Eur Biophys J 32, 703–709 (2003). https://doi.org/10.1007/s00249-003-0333-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-003-0333-8