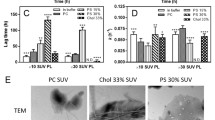
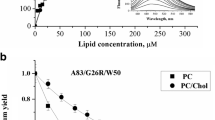
Abstract
The binding of monomeric and aggregated variants of 1–83 N-terminal fragment of apolipoprotein A-I with substitution mutations G26R, G26R/W@8, G26R/W@50 and G26R/W@72 to the model lipid membranes composed of phosphatidylcholine and its mixture with cholesterol has been investigated using fluorescent probes pyrene and Laurdan. Examination of pyrene spectral behavior did not reveal any marked influence of apoA-I mutants on the hydrocarbon region of lipid bilayer. In contrast, probing the membrane effects by Laurdan revealed decrease in the probe generalized polarization in the presence of aggregated proteins. suggesting that oligomeric and fibrillar apoA-I species induce increase in hydration degree and reduction of lipid packing density in the membrane interfacial region. These findings may shed light on molecular details of amyloid cytotoxicity.






Similar content being viewed by others
References
Fink AL (1998) Protein aggregation: folding aggregates, inclusion bodies and amyloid. Fold Des 3:R9–R23
Serpell LC (2000) Alzheimer’s amyloid fibrils: structure and assembly. Biochim Biophys Acta 1502:16–30
Gorbenko GP, Kinnunen PKJ (2006) The role of lipid–protein interactions in amyloid-type protein fibril formation. Chem Phys Lipids 141:72–82
Zerovnik E (2002) Amyloid-fibril formation. Proposed mechanisms and relevance to conformational disease. Eur J Biochem 269:3362–3371
Dobson CM (2003) Protein folding and misfolding. Nature 426:884–890
Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, Tycko R (2000) Molecular structure of β-amyloid fibril in Alzheimer’s disease brain issue. Cell 154:1257–1268
Aisenbrey C, Borowik T, Byström R, Bokvist M, Lindström F, Misiak H, Sani M, Gröbner G (2008) How is protein aggregation in amyloidogenic diseases modulated by biological membranes? Eur Biophys J 37:247–255
Arispe N, Rojas E, Pollard H (2003) Alzheimer’s disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminium. Pro Natl Acad Sci 89:10940–10944
Gorbenko GP, Trusova VM (2011) Protein aggregation in a membrane environment. Adv Protein Chem Struct Biol 84:114–142
Bucciantini M, Cecchi C (2010) Biological membranes as protein aggregation matrices and targets of amyloid toxicity. Methods Mol Biol 648:231–243
Bucciantini M, Rigacci S, Stefani M (2014) Amyloid aggregation: role of biological membranes and the aggregate-membrane system. J Phys Chem Lett 5:517–527
Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG (2005) Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem 280:17294–17300
Lashuel HA, Lansbury PT (2006) Are amyloid diseases caused by protein aggregates that mimic bacterial pore-forming toxins? Q Rev Biophys 39:167–201
Smith P, Brender J, Ramamoorthy A (2009) The induction of negative curvature as a mechanism of cell toxicity by amyloidogenic peptides. The case of islet amyloid polypeptide. J Am Chem Soc 131:4470–4478
de Planque RR, Raussen V, Contera SA, Rijkers DTS, Liskamp RMJ, Ruysschaert JM, Ryan JF, Separovic F, Watts A (2007) β-sheet structured β-amyloid (1–40) perturbs phosphatidylcholine model membranes. J Mol Biol 368:982–987
Lee CC, Sun Y, Huang H (2012) How type II diabetes-related islet amyloid polypeptide damages lipid bilayers. Biophys J 102:1059–1068
Verdier Y, Zarandi M, Penke B (2004) Amyloid beta-peptide interactions with neuronal and glial cell plasma membrane: binding sites and implications for Alzheimer’s disease. J Pept Sci 10:229–248
Sciacca MF, Brender JR, Lee DK, Ramamoorthy A (2012) Phosphatidylethanolamine enhances amyloid-fiber dependent membrane fragmentation. Biochemistry 51:7676–7684
Butterfield DA, Castegna A, Lauderback CM, Drake J (2002) Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol Aging 23:655–664
Obici L, Franceschini G, Calabresi L, Giorgetti S, Stoppini M, Merlini G, Bellotti V (2006) Structure, function and amyloidogenic propensity of apolipoprotein A-I. Amyloid 13:191–205
Rosenson RS, Brewer HB Jr, Davidson WB, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L (2012) Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 125:1905–1919
Phillips MC (2013) New insights into the determination of HDL structure by apolipoproteins: thematic review series: high density lipoprotein structure, function, and metabolism. J Lipid Res 54:2034–2048
Genschel J, Haas R, Propsting MJ, Schmidt H (1998) Apolipoprotein A-I induced amyloidosis. FEBS Lett 430:145–149
Monti DM, Guglielmi F, Monti M, Cozzlino F, Torrassa S, Relini A, Pucci P, Arciello A, Piccoli R (2010) Effects of a lipid environment on the fibrillogenic pathway of the N-terminal polypeptide of human apolipoprotein a-I, responsible for in vivo amyloid fibril formation. Eur Biophys J 39:1289–1299
Adachi E, Kosaka A, Tsuji K, Mizuguchi C, Kawashima H, Shigenaga A, Nagao K, Akaji K, Otaka A, Saito H (2014) The extreme N-terminal region of human apolipoprotein A-I has a strong propensity to form amyloid fibrils. FEBS Lett 588:389–394
Lagerstedt JO, Cavigiolio G, Roberts LM, Hong HS, Jin LW, Fitzgerald PG, Oda MN, Voss JC (2007) Mapping the structural transition in an amyloidogenic apolipoprotein A-I. Biochemistry 46:9693–9699
Chetty PS, Ohshiro M, Saito H, Dhanasekaran P, Lund-Katz S, Mayne L, Englander W, Phillips MC (2012) Effect of the Iowa and Milano mutations on the apolipoprotein A-I structure and dynamics determined by hydrogen exchange and mass spectrometry. Biochemistry 51:8993–9001
Das M, Mei X, Jayaraman S, Atkinson D, Gursky O (2014) Amyloidogenic mutations in human apolipoprotein A-I are not necessarily destabilizing—a common mechanism of apolipoprotein A-I misfolding in familial amyloidosis and atherosclerosis. FEBS J 281:2525–2542
Adachi E, Nakajima H, Mizuguchi C, Dhanasekaran P, Kawashima H, Nagao K, Akaji K, Lund-Katz S, Phillips MC, Saito H (2013) Dual role of an N-terminal amyloidogenic mutation in apolipoprotein A-I: destabilization of helix bundle and enhancement of fibril formation. J Biol Chem 288:2848–2856
Groenning M (2010) Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils—current status. J Chem Biol 3:1–18
Parasassi T, Krasnowska EK, Bagatolli L, Gratton E (1998) Laurdan and Prodan as polarity-sensitive fluorescent membrane probes. J Fluoresc 8:365–373
Girych M, Gorbenko G, Trusova V, Adachi E, Mizuguchi C, Nagao K, Kawashima H, Akaji K, Phillips M, Saito H (2014) Interaction of thioflavin T with amyloid fibrils of apolipoprotein A-I N-terminal fragment: resonance energy transfer study. J Struct Biol 185:116–124
Sanchez SA, Tricerri MA, Gratton E (2012) Laurdan generalized polarization fluctuations measures membrane packing micro-heterogeneity in vivo. Proc Natl Acad USA 109:7314–7319
Parasassi T, Stasio GD, Ravagnan G, Rusch RM, Gratton E (1991) Quantification of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescecne. Biophys J 60:179–189
Parasassi T, Stefano M, Loiero M, Ravagnan G, Gratton E (1994) Influence of cholesterol on phospholipid bilayer phase domains as detected by Laurdan fluorescence. Biophys J 66:120–132
McConnell HM, Radhakrishnan A (2003) Condensed complexes of cholesterol and phospholipids. Biochim Biophys Acta 1610:159–173
Huang J (2002) Exploration of molecular interactions in cholesterol superlattices: effect of multibody interactions. Biophys J 83:1014–1025
Dai J, Alwarawrah M, Huang J (2010) Instability of cholesterol clusters in lipid bilayers and the cholesterol’s umbrella effect. J Phys Chem B 114:840–859
Daly T, Wang M, Regen SL (2011) The origin of cholesterol’s condensing effect. Langmuir 27:2159–2161
Domanov Y, Kinnunen PKJ (2008) Islet amyloid polypeptide forms rigid lipid-protein amyloid fibrils on supported phospholipid bilayers. J Mol Biol 376:42–54
Sparr E, Engel M, Sakharov D, Sprong M, Jacobs J, de Kruijff B, Höppener J, Killian JA (2004) Islet amyloid polypeptide-induced membrane leakage involves uptake of lipids by forming amyloid fibers. FEBS Lett 577:117–120
Michikawa M, Gong JS, Fan QW, Sawamura N, Yanagisawa K (2001) A novel action of Alzheimer’s amyloid β-protein (Aβ): oligomeric Aβ promoted lipid release. J Neurosci 21:7226–7235
Zhao H, Tuominen EKJ, Kinnunen PKJ (2004) Formation of amyloid fibers triggered by phosphatidylserine-containing membranes. Biochemistry 43:10302–10307
Loura LM, do Canto AM, Martins J (2013) Sensing hydration and behavior of pyrene in POPC and POPC/cholesterol bilayers: a molecular dynamics study. Biochim Biophys Acta 1828:1094–1101
Kalyanasundaram K, Thomas JK (1977) Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc 99:2039–2044
Karpovich DS, Blanchard GJ (1995) Relating the polarity-dependent fluorescence response to vibronic coupling. Achieving a fundamental understanding of the py polarity scale. J Phys Chem 99:3951–3958
Tedeshi C, Mohwald H, Kirstein S (2001) Polarity of layer-by-layer deposited polyelectrolyte films as determined by pyrene fluorescence. J Am Chem Soc 123:954–960
Ioffe VM, Gorbenko GP (2005) Lysozyme effect on structural state of model membranes as revealed by pyrene excimerization studies. Biophys Chem 114:199–204
Kinnunen PKJ, Koiv A, Lehtonen JYA, Mustonen P (1994) Lipid dynamics and peripheral interactions of proteins with membrane surfaces. Chem Phys Lett 73:181–207
Cecchi C, Baglioni S, Fiorillo C, Pensalfini A, Liguri G, Nosi D, Rigacci S, Bucciantini M, Stefani M (2005) Insights into the molecular basis of the differing susceptibility of varying cell types to the toxicity of amyloid aggregates. J Cell Sci 118:3459–3470
Muller WE, Kirsch C, Eckert G (2001) Membrane-disordering effects of β-amyloid peptides. Biochem Soc Trans 29:617–624
Kastorna A, Trusova V, Gorbenko G, Kinnunen P (2012) Membrane effects of lysozyme amyloid fibrils. Chem Phys Lipids 165:331–337
Cecchini P, Franceschi G, Frare E, Fontana A, de Laureto P (2012) The role of tryptophan in protein fibrillogenesis: relevance of Trp7 and Trp14 to the amyloidogenic properties of myoglobin. Protien Eng Des Sel 25:199–203
Girych M, Maliyov I, Romanova M, Gorbenko G, Adachi E, Mizuguchi C, Molotkovsky J, Saito H (2013) Fluorescence energy transfer study of the lipid bilayer interactions with truncated apolipoprotein A-I mutants. Biophys Bull 29:39–50
Gautier R, Douguet D, Antonny B, Drin G (2008) HeliQuest: a web server to screen sequences with specific alpha-helical properties. Bioinformatics 24:2101–2102
Keller R (2011) New user-friendly approach to obtain an Eisenberg plot and its use as a practical tool in protein sequence analysis. Int J Mol Sci 21:5577–5591
Palgunachari MN, Mishra VK, Lund-Katz S, Phillips MC, Adeyeye SO, Alluri S, Anantharamaiah GM, Segrest JP (1996) Only the two end helixes of eight tandem amphipathic helical domains of human apo A-I have significant lipid affinity. Implications for HDL assembly. Arterioscler Thromb Vasc Biol 16:328–338
Boucrot E, Pick A, Camdere G, Liska N, Evergren E, McMahon HT, Kozlov M (2012) Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains. Cell 149:124–136
Milanesi L, Sheynis T, Xue WF, Orlova EV, Hellewell AL, Jelinek R, Hewitt EW, Radford S, Saibil HR (2012) Direct three-dimensional visualization of membrane disruption by amyloid fibrils. Proc Natl Acad Sci 109:20455–20460
Acknowledgments
This work was supported by the grant from Fundamental Research State Fund of Ukraine (project number F54.4/015) and Grant-in-Aid for Scientific Research 25293006 (to H.S.) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trusova, V., Gorbenko, G., Girych, M. et al. Membrane Effects of N-Terminal Fragment of Apolipoprotein A-I: A Fluorescent Probe Study. J Fluoresc 25, 253–261 (2015). https://doi.org/10.1007/s10895-015-1501-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1501-9