Abstract
As one of the important biodiversity conservation areas in China, the ecosystem in the lower reaches of the Yarlung Zangbo River is fragile, and is particularly sensitive to global changes. To reveal the diversity pattern of phytoplankton, the metabarcode sequencing was employed in the Medog section of the lower reaches of the Yarlung Zangbo River during autumn 2019 in present study. The phytoplankton assemblies can be significantly divided into the main stem and the tributaries; there are significant differences in the phytoplankton biomass, alpha and beta diversity between the main stem and the tributaries. While both the main stem and the tributaries are affected by dispersal limitation, the phytoplankton assemblages in the entire lower reaches are primarily influenced by heterogeneous selection. Community dissimilarity and assembly process were significantly correlated with turbidity, electrical conductivity, and nitrogen nutrition. The tributaries were the main source of the increase in phytoplankton diversity in the lower reaches of the Yarlung Zangbo River. Such diversity pattern of phytoplankton in the lower reach may be caused by the special habitat in Medog, that is, the excessive flow velocity, and the significant spatial heterogeneity in physical and chemical factors between stem and tributaries. Based on the results and conclusions obtained in present study, continuous long-term monitoring is essential to assess and quantify the impact of global changes on phytoplankton.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytoplankton plays a crucial role as primary producers in aquatic ecosystems, influencing energy flow and biogeochemical cycles in water ecosystems. The community diversity of phytoplankton is closely related to the physicochemical environment and hydrological conditions of water bodies, providing a visual reflection of the impact of climate change or human activities on aquatic ecosystems [36]. The formation and driving factors of phytoplankton diversity patterns are generally considered to be determined by four processes similar to other types of microorganisms: speciation, selection, drift, and dispersal [30]. These processes interact and collectively shape the global or regional patterns of phytoplankton on different temporal and spatial scales. Most classical theoretical models, experimental simulations, and field studies suggest that in river ecosystems, the diversity patterns of phytoplankton are primarily influenced by the processes of dispersal and selection [25]. The relative strength of dispersal and selection processes in phytoplankton assemblies in different flow rate water bodies often changes during different stages of community succession. In fast-flowing rivers, dispersal limitation usually dominates the early stages of community succession. However, in later stages, both selection and dispersal limitation processes play significant roles [2]. Furthermore, spatial scale is also one of the factors influencing community and community assembly processes [5].
The Yarlung Zangbo River, China’s largest river on the Tibetan Plateau, meanders over 2200 km, exhibiting diverse ecosystems across its upper, middle, and lower reaches. Characterized by wide plateaus and a high plateau semi-arid climate, the upper and middle reaches contrast with the lower reaches, forming a canyon river with swift currents and a tropical rainforest climate. Medog, located in the lower reaches, contributes to the unique biodiversity of the Yarlung Zangbo. Tributaries like Jinzhu Zangbo and Yanglang Zangbo originate from glaciers, shaping a distinctive river landscape from icy zones to subtropical and tropical zones. This geological and climatic diversity fosters a unique biodiversity pattern and a distinct aquatic ecosystem in the Medog (Yang 1983). Due to the variation in important environmental factors such as water, heat, and light, altitude gradient has become a significant aspect in the study of biodiversity patterns [12]. Thus, the Medog section of the Yarlung Zangbo River exhibits a substantial altitude drop over a short distance, rendering its ecosystems highly sensitive to human activities and climate change [7, 28]. While biodiversity studies in the downstream region mainly focus on terrestrial ecosystems, aquatic organisms, particularly phytoplankton, remain understudied [18, 20, 21, 39]. Addressing this research gap is essential for a comprehensive understanding of the Yarlung Zangbo’s fragile and dynamic aquatic ecosystems.
In recent years, the impact of global changes on plateau ecosystems has become increasingly evident. Influenced by the warming and humidifying climate on the plateau, the upstream inflow of the Yarlung Zangbo River has significantly increased. Simultaneously, human activities such as road construction along the river and hydropower development have led to noticeable alterations in the hydrological conditions of certain sections of the main stem and tributaries of the Yarlung Zangbo [37]. Against this backdrop, understanding the baseline phytoplankton diversity of the main stem and tributaries in the Medog segment of the Yarlung Zangbo is fundamental for regional aquatic biological resource conservation. Additionally, all main stems and tributaries of the Yarlung Zangbo within Medog are characterized by canyon river flow, where high water velocities impede the upstream dispersion of numerous aquatic organisms. This flow pattern also restricts the exchange of aquatic biological assemblies among different tributaries, and the horizontal movement between the main stem and tributaries may primarily occur in a unidirectional manner. Therefore, analyzing and characterizing the eukaryotic phytoplankton diversity patterns in this typical aquatic habitat is of significant theoretical importance for understanding the mechanisms governing the formation and maintenance of eukaryotic phytoplankton community diversity.
In recent years, the Uthermoll sedimentation method and microscopic observation for algae identification have encountered numerous challenges due to the phenotypic plasticity and cryptic diversity of algae [40]. Metabarcoding sequencing offers an improved alternative to overcome these limitations associated with traditional microscopic identification techniques (Blaxter, 2004). In this study, high-throughput DNA barcoding sequencing technology was employed to conduct a systematic analysis of the phytoplankton assemblies in the main stem and major tributaries of the Yarlung Zangbo in the Medog segment. The aim was to reveal the distribution patterns of phytoplankton in the Yarlung Zangbo Medog segment, identify the primary influencing factors, and understand the mechanisms sustaining these patterns. This research provides a scientific basis for the rational conservation and sustainable development of aquatic biological resources in the downstream Yarlung Zangbo.
Materials and Methods
Phytoplankton Sampling and Evaluation of Environmental Variables
To represent diverse hydrological dynamics and urban development conditions along the downstream Yarlung Zangbo River and to achieve a balance between water characteristics and road accessibility, a total of 14 sampling sites were selected during 14–27 October 2019. Seven points were designated along the main stem, with an additional seven points along various tributaries (Fig. 1a). All water samples were pre-sieved using a plankton net with a pore diameter of approximately 180 μm to remove large planktonic animals and suspended solids. Three liters of water were collected at each site and then transported to the laboratory. Environmental DNA samples were enriched using a 0.22-μm polycarbonate filter (Millipore) with a 1-l water. Chlorophyll-a concentration (Chla) was used to estimate phytoplankton biomass, measured by filtering 1 l of water through a GF/C membrane (with a diameter 0.45 μm, Waterman). All filters were stored in liquid nitrogen for further analysis. The OMEGA water DNA kit (D5525-01, OMEGA biotech) was used for DNA extraction. An ultra-micro spectrophotometer (Nanodrop 8000, Thermo) was used to test the quality of extracted total DNA. The universal eukaryotic V4 SSU rRNA region primers (forward primer CCAGCASCYGCGGTAATTCC, reverse primer ACTTTCGTTCTTGATYRA) were used [29]. The constructed libraries were tested by Qsep-400 and then sequenced on Hiseq 2500 (Illumina). All raw data were uploaded to the Sequence Read Archive (SRA, National Center for Biotechnology Information).
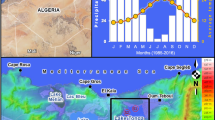
a Site distribution in the Medog segment of the Yarlung Zangbo river, showing habitat images of typical main stem and tributaries. Pink dots and blue dots represent main stem sampling sites and tributary sampling sites respectively, the red arrows show the flow direction; b Spearman correlation analysis of physicochemical index and nutrient concentrations in the downstream of the Yarlung Zangbo river, the size and color of circle represent correlation ship; c Comparison of various physicochemical index and nutrient concentrations between the main stem and tributaries in the Medog segment of the Yarlung Zangbo river, the *, **, and *** represent p values < 0.05, < 0.01, and < 0.001, respectively
A portable multi-parameter water quality analyzer (Hydrolab, Hash) was used in filed for measuring physicochemical index like water temperature (Kelvin temperature), dissolved oxygen (DO), pH, and electronical conductivity (EC). Turbidity (Turb) was measured with a portable turbidity meter (2100Q, Hash). Other environmental factors such as chlorophyll-a concentration (Chla), total nitrogen (TN), ammonium nitrogen (NH4-N), total phosphorus (TP), and soluble reactive phosphorus (SRP) were determined following the “Methods for the Monitoring and Analysis of Water and Wastewater” (Fourth Edition) [35]. The variance inflation factor (VIF) in regression analysis was used to detect the multicollinearity across environmental variables, and variables with VIF values greater than 10 were deleted in following analysis.
Sequencing and Annotation of Metabarcoding Data, Statistical Analysis
The raw data from meta-barcoding sequencing were primarily processed using Usearch (v11.0.667) for filtering, quality control, primer trimming, merging, chimera removal, and OTU clustering [11]. Sequences with a similarity of ≥ 97% were grouped into operational taxonomic units (OTUs) using USEARCH (v10.0). OTUs with abundances lower than 0.005% were filtered out, and chimera removal was performed using UCHIME (version 8.1) [10]. The representative sequences of each OTU were annotated with USEARCH (v10.0), and only OTUs corresponding to eukaryotic algae were further analyzed [9]. Their community matrix was rarefied based on the sample with the lowest sequencing depth. The resulting sequence matrix was aligned with MAFFT, trimmed using TrimAl, and used to construct a maximum likelihood tree with IQtree [23]. The majority consensus tree was then employed for calculating alpha and beta phylogenetic diversity, and analyzing community assembly processes. The community matrix was first Hellinger transformed, and environmental variables matrix (except pH) was log(x + 1) transformed using VEGAN package. Alpha diversity (Shanno-Wiener, and phylogenetic diversity indices based on whole tree phylogenetic distance) and principal coordinate analysis (PCoA) were performed using Vegan and PICANTE packages [17, 24]. Beta diversity (Sorensen dissimilarity and phylogenetic Sorensen dissimilarity) was calculated and further divided into species replacement and richness difference components based on both taxonomic and phylogenetic β-diversity using betapart and adespatial packages [1, 8]. βNTI and RCbray values were calculated using picante package, and assembly processes were assessed and quantified with iCAMP package (Stegen et al., 2013). The contribution percentage of homogeneous selection (HoS) was determined based on the percentage of pairwise comparisons with βNRI values less than − 1.96, while heterogeneous selection (HeS) was determined based on those with βNRI values greater than 1.96. Additionally, the contribution percentage of homogenizing dispersal (HD) was calculated from the percentage of pairwise comparisons with |βNRI|≤ 1.96 and RC < − 0.95, while dispersal limitation (DL) was determined from those with|βNRI|≤ 1.96 and RC > 0.95. Ecological drift (ED) was assessed based on the percentage of pairwise comparisons with |βNRI|≤ 1.96 and |RC|≤ 0.95 [22]. Sink-source analysis of phytoplankton community composition along the main stem was traced with the FEAST package [27]. In the sink-source analysis, we focused solely on downstream points located between adjacent main stem sampling sites where investigated tributaries merged during this survey,therefore, points MT06 and MT04 were excluded (Fig. 1a). Correlations among environmental factors, spatial distance, beta diversity, and community assembly processes (βNTI) were explored using the mantel test in VEGAN package. All analyses were conducted in R.
Results
Water Environmental Variables
Spearman correlation analysis revealed a significant correlation (r > 0.8) among pH, EC, and NO3-N in the Medog segment of the Yarlung Zangbo River. Strong correlations were also observed between TN and NH4-N, SRP and DO, as well as TURB and pH, EC, and NO3-N (Fig. 1b). In comparing environmental factors between the main stem and tributaries, significant differences were found in pH, EC, Turb, NH4-N (p < 0.001), and TP (p < 0.05) (Fig. 1c). Subsequently, a variance inflation factor (VIF) analysis was conducted, and pH and NH4-N were removed from the subsequent analysis based on VIF values below 10.
Alpha Diversity and Biomass Distribution
A total of 564 eukaryotic algae OTUs from Chlorophyta, Charophyta, Cryptophyta, Dinoflagellata, and Ochrophyta were identified. Further detailed classification was conducted due to the high richness and abundance observed in Ochrophyta, including taxa such as Chrysophyceae, Eustigmatophyceae, Dictyochophyceae, Diatomea, and others. Both main stem and tributaries showed high richness in Chrysophyceae, Chlorophyta, and Diatomea, followed by Dinoflagellata (Fig. 2a). The main stem generally exhibited significantly higher richness in Chrysophyceae, Chlorophyta, Diatomea, Dinoflagellata, and Cryptophyta than tributaries (Fig. 2a). Most sites were dominated by Chrysophyceae and Chlorophyta, with some exceeding 60% relative abundance (Fig. 2b). Principal coordinates analysis (PCoA) based on Bray–Curtis distances revealed a significant difference between main stem and tributaries (R2 = 0.26, p = 0.001), with higher dissimilarity among tributaries (Fig. 2c). There is significant differences alpha diversity between the main stem and tributaries. Both Shannon–Wiener and phylogenetic diversity indices showed higher in main stem sites. In contrast, the biomass showed significantly higher in the tributaries than main stem (Fig. 2d).
a Number of detected OTUs at each sampling site; b relative abundance of each algal group at each sampling site; c PCoA of 14 assemblies based on Bray–Curtis Distances, with adonis analysis r2 = 0.26 and p = 0.001; d comparison of taxonomic alpha diversity, phylogenetic alpha diversity, and biomass (Chla concentration) between the main stem and tributaries. Red words or red circles represent main stem sampling sites, while blue words or blue triangles represent sampling sites in tributaries. The *, **, and *** represent p values < 0.05, < 0.01, and < 0.001, respectively
Beta Diversity
Phytoplankton assemblies in both tributaries and the main stem were dominated by turnover either in taxonomic or phylogenetic beta diversity (Fig. 3a). The taxonomic Sorensen dissimilarity and phylogenetic Sorensen dissimilarity in tributaries were significantly higher than those in the main stem. Both the species turnover and richness difference of the Sorensen dissimilarity and the phylogenetic Sorensen dissimilarity in in tributaries were notably higher than those in the main stem (Fig. 3b, c).
Comparison of taxonomic and phylogenetic Sorensen dissimilarity, as well as components (species replacement and richness difference). a Composition of taxonomic beta diversity and phylogenetic beta diversity; b Comparison of taxonomic Sorensen dissimilarity between the main stem and tributaries; c Comparison of phylogenetic Sorensen dissimilarity between the main stem and tributaries
Assembly Processes and Source Analysis
Overall, the phytoplankton assemblies were influenced by four processes: heterogeneous selection, homogeneous selection, dispersal limitation, and ecological drift. Heterogeneous selection has the highest proportion, with the ration up to 0.63 (Fig. 4a). The phytoplankton assemblies only in the main stem or tributaries are influenced by three processes: heterogeneous selection, ecological drift, and dispersal limitation. The proportion of heterogeneous selection is consistent in both the main stem and tributaries, with a value of 0.14. Dispersal limitation has proportions of 0.72 in the main stem and 0.76 in tributaries. The proportion of ecological drift is 0.14 in the main stem and 0.10 in tributaries (Fig. 4a).
Planktonic algal sources were classified into three parts: main stem, tributary, and unknown. The analysis results for the five main stem sampling points indicated that there were no significant differences in the proportion of unknown sources among these points, ranging from 0.16 to 0.27. The proportion of planktonic algae originating from the upstream main stem point varied from 0.57 to 0.84 (Fig. 4b). With an increasing number of studied tributaries, the proportion of main stem sources showed a decreasing trend. From upstream to downstream, there was a gradual increase in the proportion of planktonic algae originating from the tributaries (Fig. 4b).
Driving Factors
The taxonomic beta diversity of planktonic algae in both the main stem and tributaries appears unaffected by most physicochemical factors, except pH. Geographical distances between sampling sites also show no significant correlation (Fig. 5). However, taxonomic Sorensen dissimilarity across all assemblies’ correlates significantly with EC (r = 0.2541, p = 0.047) and Turb (r = 0.6722, p = 0.001). This correlation is mainly attributed to the impact of EC (r = 0.2322, p = 0.05) and Turb (r = 0.6605, p = 0.001) on richness difference. The phylogenetic Sorensen dissimilarity of both main stem (r = 0.4244, p = 0.032) and tributaries (r = 0.6928, p = 0.001) significantly correlates with EC. However, there is a distinction in how EC affects main stem and tributaries assemblies. EC influences species replacement in main stem (r = 0.4005, p = 0.045), while affecting the richness difference (r = 0.4651, p = 0.039) in tributaries. Considering both tributaries and the main stem together, phylogenetic Sorensen dissimilarity primarily correlates with EC (r = 0.3065, p = 0.029) and Turb (r = 0.6919, p = 0.001), consistent with taxonomic Sorensen dissimilarity results. Notably, EC significantly influences the species replacement (r = 0.2513, p = 0.013) of overall phylogenetic Sorensen dissimilarity (Fig. 5). The βNTI of main stem assemblies shows no significant correlation with water physicochemical factors, nutrient concentrations, or geographical distances between sampling points. In contrast, the βNTI of tributaries significantly correlates with DO (r = 0.7872, p = 0.001), EC (r = 0.5187, p = 0.047), and TN (r = 0.6123, p = 0.012). Overall, community assembly processes in the main stem and tributaries of the Yarlung Zangbo river in the Medog section are significantly correlated with EC (r = 0.2904, p = 0.027) and Turb (r = 0.6146, p = 0.001) (Fig. 5).
Discussion
Biomass and Species Distribution Between Main Stem and Tributaries
Despite the extremely low phytoplankton biomass levels in both the main stem and tributaries of the Medog section of the Yarlung Zangbo River, the biomass in tributaries remains significantly higher than that in the main stem. We hypothesize that this phenomenon is primarily driven by Turb. Studies indicate a positive correlation between water turbidity and algal biomass within a certain range, and excessively high turbidity and suspended particulate matter (TSS) can inhibit algal growth [4]. In this study, turbidity in the main stem is relatively high, while most tributaries have extremely low turbidity levels. This allows phytoplankton in tributaries to access more sunlight for photosynthesis, resulting in higher Chla concentrations in tributaries than in main stem. Although tributaries seem more conducive to algal growth from the perspective of Turb, the species richness of phytoplankton in the main stem of the Medog section is significantly higher than that in tributaries. We speculate that this phenomenon is likely due to the unique hydrological characteristics of the Medog section of the Yarlung Zangbo River. The strong water flow in the main stem hinders the dispersal of phytoplankton and their remnants upstream. Consequently, the main stem functions more like a large species pool, collecting phytoplankton and remnants from various upstream tributaries [32]. The use of eDNA methods facilitates the detection of a greater species diversity in the main stem. Source tracing analysis of phytoplankton in the main stem supports this speculation, indicating that including more tributaries increases the proportion of tributary components in the main stem.
Beta Diversity and Driving Factors
In general, beta diversity is mainly shaped by species turnover (replacement) and differences in species richness (nestedness) between different assemblies 15. With increasing spatial scale and gradients, environmental heterogeneity typically changes, leading to variations in species richness and species turnover between assemblies, further increasing dissimilarity between assemblies [16]. In current study of the Yarlung Zangbo River, turnover significantly contributed to beta diversity either in main stem or tributaries. Taxonomic beta diversity of phytoplankton was not significantly correlated with measured environmental factors in either the main stem or tributaries. However, when considering the entire Yarlung Zangbo system, overall taxonomic beta diversity showed a significant correlation with conductivity and water turbidity. These factors mainly influenced the nestedness process and overall beta diversity. This correlation is likely attributed to notable gradient differences in conductivity and water turbidity between the main stem and tributaries, along with distinct species composition variations among different sampling points [13]. Additionally, tributaries exhibited higher environmental heterogeneity compared to the main stem, resulting in greater community dissimilarity. These findings suggest the potential existence of algae in the Medog section of the Yarlung Zangbo River that are highly specialized to specific habitats (such as conductivity and water turbidity) and may be unique to certain tributaries [20, 21, 32].
Unlike taxonomic beta diversity, phylogenetic beta diversity in both main stem and tributaries were notably correlated with EC in the Medog section of the Yarlung Zangbo River. EC primarily drove the species replacement in the main stem assemblies, while it predominantly influenced the richness difference in tributaries. This implies that differences among tributaries result from conductivity-induced variations in species richness, whereas differences among main stem assemblies mainly from turnover [32]. Significant environmental factors affecting phytoplankton community phylogenetic beta diversity included Turb and EC. Similar to taxonomic beta diversity, Turb mainly influenced the richness difference between main stem and tributary sampling points, while EC impacted the species replacement between these points. This suggests that Turb disrupts species numbers in the Medog section, leading to the loss of certain species due to changes in water turbidity. Conversely, EC and nitrate nitrogen influence species composition, implying a potential competition or selection within the phytoplankton community in response to changes in water conductivity and nitrate nitrogen.
Spatial correlation as an important factor in community ecology is typically taken into account [6]. Normally, phytoplankton assemblies in the main stem should exhibit a significant correlation with hydrological distance when tributary inflow is not considered or inflow is relatively uniform. However, the present results did not support this hypothesis, suggesting that it might be due to the non-uniformity of tributary inflow. The varying number and water volume of tributaries, along with the quantity of phytoplankton (and their remnants) they carry, are challenging to equalize within the same distance. This leads to a lack of a significant correlation between community dissimilarity in the main stem phytoplankton and spatial distance [26]. Additionally, the consistent addition of some components of phytoplankton assemblies from unknown sources in the main stem is also a partial reason for this result.
Assembly Processes and Mechanisms
The balance between niche-based and stochastic processes in community assembly often changes with hydrological patterns; for instance, plankton assemblies in rivers with high flow rates, such as those in mountain streams, tend to have a higher proportion of stochastic processes [2]. Dispersal limitation can lead to turnover processes between different assemblies, increasing dissimilarity between assemblies [19]. In this study, the assembly processes of different assemblies within the main stem and between tributaries were both dominated by dispersal limitation. This is consistent with the conclusion drawn from beta diversity analysis that turnover between assemblies dominates both in the main stem and between tributaries, indicating that the main reason for community differences between main stem and tributaries is species replacement between different assemblies [33]. This conclusion aligns with the unique habitat characteristics of the Medog section of the Yarlung Zangbo River. The high flow rate restricts bidirectional flow of phytoplankton. Additionally, different tributaries are significantly affected by environmental factors such as EC, DO, and nitrogen nutrients (TN), leading to differences in phytoplankton community composition between them. In contrast, main stem sampling points show similarity both upstream and downstream. However, as more adjacent main stem sampling points include different tributaries, their community similarity decreases. Although the assembly processes within tributaries and main stems are dominated by dispersal limitation, the overall phytoplankton community assembly process in the Medog section of the Yarlung Zangbo River is mainly determined by deterministic processes (heterogeneous selection).
Conclusion and Outlook
The phytoplankton in the Medog section of the Yarlung Zangbo River exhibits a unique and diverse pattern, especially evident in three aspects: (1) alpha diversity in tributaries is significantly lower than in the main stem; however, biomass in tributaries is significantly higher than main stem; (2) beta diversity and its components in tributaries shows significantly higher than the main stem; (3) the Medog section of the Yarlung Zangbo River is mainly governed by deterministic processes overall, and both main stems and tributaries are dominated by dispersal limitation. The unique pattern of phytoplankton diversity in the Medog section is attributed to the distinctive river habitats, characterized by significant differences in water turbidity, conductivity, and nitrogen nutrients between main stems and tributaries, as well as the short-distance vertical drops and fast water flow in rivers within Medog, hindering the reverse dispersal and exchange of phytoplankton and their remnants in these water bodies.
Global changes, including human activities and climate change, are the primary factors affecting regional ecosystems of Medog. Changes in water flow from climate change in the upstream of the Yarlung Zangbo River and changes in hydrological conditions caused by human activities (such as road and dam construction) are likely to alter the regional river habitat characteristics in the Medog section. This will, in turn, impact aquatic organisms, especially phytoplankton. From the conclusions drawn in this study, continuous long-term monitoring is essential to assess and quantify the impact of climate change and human activities on aquatic organisms, particularly phytoplankton, in the Medog. Protection of the aquatic biodiversity in the downstream Yarlung Zangbo River should primarily focus on tributary waters.
Data Availability
The raw data of all samples generated in present work are available in the Sequence Read Archive (SRA, National Center for Biotechnology Information) under the accession number PRJNA1092358.
References
Baselga A, Orme CDL (2012) Betapart: an R package for the study of beta diversity. Methods Ecol Evol 3:808–812
Borics G, Abonyi A, Salmaso N, Ptacnik R (2021) Freshwater phytoplankton diversity: models, drivers and implications for ecosystem properties. Hydrobiologia 848:53–75
Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25(15):1972–1973
Chaffin JD, Kane DD, Stanislawczyk K, Parker EM (2018) Accuracy of data buoys for measurement of cyanobacteria, chlorophyll, and turbidity in a large lake (Lake Erie, North America): implications for estimation of cyanobacterial bloom parameters from water quality sonde measurements. Environ Sci Pollut Res 25:25175–25189
Chase JM (2014) Spatial scale resolves the niche versus neutral theory debate. J Veg Sci 25:319–322
Dormann CF, McPherson JM, Araújo MB, Bivand R, Bollinger J, Carl G, Davies RG, Hirzel A, Jetz W, Kissling WD, Kühn I, Ohlemüller R, Peres-Neto PR, Reineking B, Schröder B, Schurr FM, Wilson R (2007) Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30:609–628
Deng LB, Chen DL, Deng LQ (2011) Ecological evaluation of Yarlung Zangbo Grand Canyon National Nature Reserve in Tibet. Scientia Silvae Sinicae 47(5):1–6 in Chinese with English abstract
Dray S, Blanchet G, Borcard D, Guenard G, Jombart T, Larocque G, Legendre P, Madi N, Wagner HH (2018) Package ‘adespatial’. Available: https://cran.microsoft.com/web/packages/adespatial/adespatial.pdf. Accessed 7 Nov 2023
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26(19):2460–2461
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27(16):2194–2200
Edgar RC, Flyvbjerg H (2015) Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics 31(21):3476–3482
Gaston KJ (2000) Global patterns in biodiversity. Nature 405:220–227
Hu A, Ren M, Wang J (2021) Microbial species performance responses to environmental changes: genomic traits and nutrient availability. Ecology 102:e03382
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20(4):1160–1166
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 19(1):134–143
Kraft NJ, Comita LS, Chase JM, Sanders NJ, Swenson NG, Crist TO, Stegen JC, Velland M, Boyle B, Anderson MJ, Cornell HV, Davies KF, Freestone AL, Inouye BD, Harrison SP, Myers JA (2011) Disentangling the drivers of β diversity along latitudinal and elevational gradients. Science 333:1755–1758
Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26(11):1463–1464
La Q, Zhaxi CR, Zhu WD, Xu M, Zhong Y (2014) Plant species-richness and association with environmental factors in the riparian zone of the Yarlung Zangbo River of Tibet, China. Biodivers Sci 22(3):337–347 in Chinese with English abstract
Lewthwaite JMM, Debinski DM, Kerr JT (2017) High community turnover and dispersal limitation relative to rapid climate change. Glob Ecol Biogeogr 26(4):459–471
Li L, Ma B, Jin X, Jin HY, Wu S, Chen ZX, Cheng L, Wang NM, Hao QR (2022) Structural and diversity characteristics of fish assemblies in the Medog reach of the Yarlung Zangbo Grand Canyon. J Fish Sci China 29(9):1326–1336 in Chinese with English abstract
Li ZF, Jiang XM, Wang J, Meng XL, Zhang JQ, Xie ZC (2022) Species diversity and driving factors of benthic macroinvertebrate assemblages in the middle and lower reaches of the Yarlung Zangbo River. Biodivers Sci 30(6):1–13 in Chinese with English abstract
Ning D, Yuan M, Wu L, Zhang Y, Guo X, Zhou X, Yang Y, Arkin AP, Firestone MK, Zhou J (2020) A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat Commun 11:4717
Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32(1):268–274
Oksanen J, Blanchet F, Kindt R, Legendre P, Minchin P, O’Hara R, Simpson G, Solymos P, Stevens M, Wagner H (2017) Vegan: community ecology package. R package version 2.3–5
Pearson DE, Ortega YK, Eren O, Hierro JL (2018) Community assembly theory as a framework for biological invasions. Trends Ecol Evol 33:313–325
Picazo F, Vilmi A, Aalto J, Soininen J, Casamayor EO, Liu Y, Wu Q, Ren L, Zhou J, Shen J, Wang J (2020) Climate mediates continental scale patterns of stream microbial functional diversity. Microbiome 8:92
Shenhav L, Thompson M, Joseph TA, Briscoe L, Furman O, Bogumil D, Mizrahi I, Peer I, Halperin E (2019) FEAST: fast expectation-maximization for microbial source tracking. Nat Methods 16:627–632
Shi XY, Li XY, Wei CY, Sun G, Liu Z, Zhao X, Zhou JD, Fan J, Li C, Lü Z (2023) Avian and mammal diversities and their altitudinal and seasonal distribution patterns in Yarlung Zangbo Grand Canyon, China. Biodivers Sci 31(2):1–13 in Chinese with English abstract
Stoeck T, Bass D, Nebel M, Christen R, Jones MDM, Breiner H-W, Richards TA (2010) Multiple marker parallel tag environmental DNA sequencing reveals a highly complex eukaryotic community in marine anoxic water. Mol Ecol 19(s1):21–31
Vellend M (2010) Conceptual synthesis in community ecology. Q Rev Biol 85:183–206
Wang XX, Liu LL, Yang XF, Yang RB, Liu HP (2022) Using periphyton algae to assess stream conditions of Yarlung Zangbo river basin. Acta Hydrobiol Sin 46(12):1816–1831 in Chinese with English abstract
Wang J, Meier S, Soininen J, Casamayor E, Tang X, Yang X, Zhang Y, Wu Q, Zhou J, Shen J (2017) Regional and global elevational patterns of microbial species richness and evenness. Ecography 40(3):393–402
Wang J, Legendre P, Soininen J, Yeh CF, Graham EB, Stegen JC, Casamayor EO, Zhou J, Shen J, Pan P (2020) Temperature drives local contributions to beta diversity in mountain streams: stochastic and deterministic processes. Glob Ecol Biogeogr 29:420–432
Wasmund N, Topp I, Schories D (2006) Optimising the storage and extraction of chlorophyll samples. Oceanologia 48(1):125–144
Wei FS (2002) The monitoring and analysis methods of the water and waste water (Fourth version). China Environmental Science Press, Beijing in Chinese
Winder M, Sommer U (2012) Phytoplankton response to a changing climate. Hydrobiologia 698:5–16
Xu ZX, Ban CG, Zhang R (2022) Evolution laws and attribution analysis in the Yarlung Zangbo River basin. Adv Water Sci 33(4):519–530 in Chinese with English abstract
Yang YC, Li BY, Yin ZS et al (1983) Geomorphology in Tibet. Beijing: Science Press, 1–238. in Chinese
Yu D, Zhang Z, Zhang J, Lin PC, Xiong SR, Tang FL, Liu HZ (2019) Genetic diversity and population demography of Schizothorax molesworthi from the Medog area of lower reaches of the Yarlung Zangbo river and Lohit river. Acta Hydrobiol Sin 43(5):923–930 in Chinese with English abstract
Zhu H, Xiong X, Liu BW, Liu GX (2024) Lakes-scale pattern of eukaryotic phytoplankton diversity and assembly process shaped by electrical conductivity in central Qinghai-Tibet Plateau. FEMS Microbiol Ecol 100:1–9
Acknowledgements
We would like to express our gratitude to Dr. Ren Zhu and Dr. Baoqiang Wang from the Institute of Hydrobiology, Chinese Academy of Sciences, for their assistance during field sampling in Medog.
Funding
This work was supported by the Second Tibetan Plateau Scientific Expedition and Research Program (STEP, Grant No. 2019QZKK0304) and the Youth Innovation Promotion Association CAS (2023355).
Author information
Authors and Affiliations
Contributions
Huan Zhu: conceptualization, methodology, investigation, data curation and analysis, visualization, original draft. Shuyin Li: investigation. Xiong Xiong, Pengcheng Lin and Dekui He: investigation and data curation. Benwen Liu: data curation and analysis. Guoxiang Liu: funding acquisition and supervision validation. All authors have agreed to submit and publish this work.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, H., Li, S., Wu, Z. et al. Diversity Patterns of Eukaryotic Phytoplankton in the Medog Section of the Yarlung Zangbo River. Microb Ecol 87, 59 (2024). https://doi.org/10.1007/s00248-024-02371-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00248-024-02371-6