Abstract
In aquatic environments, Vibrio and cyanobacteria establish varying relationships influenced by environmental factors. To investigate their association, this study spanned 5 months at a local shrimp farm, covering the shrimp larvae stocking cycle until harvesting. A total of 32 samples were collected from pond A (n = 6), pond B (n = 6), effluent (n = 10), and influent (n = 10). Vibrio species and cyanobacteria density were observed, and canonical correspondence analysis (CCA) assessed their correlation. CCA revealed a minor correlation (p = 0.847, 0.255, 0.288, and 0.304) between Vibrio and cyanobacteria in pond A, pond B, effluent, and influent water, respectively. Notably, Vibrio showed a stronger correlation with pH (6.14–7.64), while cyanobacteria correlated with pH, salinity (17.4–24 ppt), and temperature (30.8–31.5 °C), with salinity as the most influential factor. This suggests that factors beyond cyanobacteria influence Vibrio survival. Future research could explore species-specific relationships, regional dynamics, and multidimensional landscapes to better understand Vibrio-cyanobacteria connections. Managing water parameters may prove more efficient in controlling vibriosis in shrimp farms than targeting cyanobacterial populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shrimp farming is one of the most significant products for the Malaysian aquaculture industry. Among the Association of Southeast Asian Nations (ASEAN) countries, Malaysia has maintained its competitiveness in exporting fresh shrimp, alongside Thailand [1]. In Sarawak, the state’s Modernization of Agriculture and Regional Development Minister aims to expand shrimp farming with the goal of producing RM 1 billion in exports by 2030 (Sulok [2]). The extensive shrimp farm selected for this study achieved the production of 165 mt of shrimp in 2021 [3], which marks great potential for the goal. As shrimp farming in Malaysia has the potential to be profitable and contribute to the country’s economic expansion, effective disease prevention measures are essential to avoid profit loss from bacterial infections.
The shrimp farming industry regards vibriosis as a significant threat as it can affect the entire life cycle of shrimp, ranging from eggs to brood stock, which may lead to the complete eradication of an entire population in case there is an outbreak [4, 5]. These infections can spread rapidly within shrimp ponds due to the proximity and crowded conditions in aquaculture settings [6]. The Vibrioneceae family has captivated scientists due to its adaptability in the aquaculture environment. In the marine ecosystem, their prevalence and pathogenicity in the environment raise concerns, as Vibrio is still endemic in certain countries [7]. They were also recognized to interact with other bacterial groups [8] and other aquatic creatures in the context of an aquatic environment [9]. Vibrio species possess a curved morphology and exhibit flagellated motility on one end, measuring 0.5–0.8 μm in width and 2–3 μm in length [10]. In clinical microbiology and food safety, V. cholerae, V. parahaemolyticus, V. vulnificus, V. alginolyticus, and V. mimicus are among the Vibrio species that are potentially dangerous [11].
It has been demonstrated through previous research that cyanobacteria play a crucial role in the persistence of Vibrio in our environment [12]. These bacteria possess the unique ability to conduct photosynthesis, and they exist in either coccoid unicellular, coccoid multicellular, or filamentous forms [13]. Certain cyanobacterial species coexist in habitats alongside Vibrio, providing dissolved organic matter that sustains the Vibrio community [14]. These bacteria, classified as opportunistic in nature, exhibit rapid proliferation in specific conditions such as low oxygen levels, insufficient nourishment, and contaminated water [15]. These environmental stressors, resulting from the opportunistic behavior of both cyanobacteria and Vibrio, can also adversely impact shrimp. As microorganisms are very capable of fostering symbiotic relationships with each other, plants, and animals, more research is required to better understand them.
Despite the evidence of their association, there have been a number of studies that have demonstrated contradictory results. Some isolated strains of cyanobacteria have been found to exhibit antibacterial properties against pathogenic microorganisms, indicating that they can potentially eradicate Vibrio species instead of nurturing them [16]. Another study has found that the relationship between cyanobacteria and Vibrio species depends on the growth stage of the cyanobacteria, which indicates that the association is not without specific circumstances [17]. To accommodate the differences in findings, there is a need to conduct research on the interaction between cyanobacteria and Vibrio species, particularly in the context of shrimp farms, where favorable conditions for the proliferation of both bacteria genera are naturally present.
Aside from the potential association with cyanobacteria, water parameters such as temperature, salinity, and pH can also influence the growth and abundance of opportunistic Vibrio species [18] in shrimp farms. High water temperatures, for example, were proven to provide optimal conditions for the growth of Vibrio species [19]. Shrimps are equally sensitive to changes in their environment, and stressors such as suboptimal water quality can weaken their immune systems [20], making them more susceptible to infections from Vibrio species. On the contrary, the maintenance of optimum salinity can lead to better survival, weight gain, and protein retention rates in reared shrimp [21].
To find out the correlation between both Vibrio species and cyanobacteria with water parameters, a statistical method called canonical correspondence analysis (CCA) was employed. Previous studies have used CCA as a tool to analyze the microbial communities of aquatic environments, and it has been effective in elucidating the correlation between Vibrio populations, particulate organic matter, and environmental factors [22]. By implementing CCA as the statistical method, the microbial ecology consisting of the association between both genera and the water parameters was studied.
This study is focused on the aquaculture of shrimp and encompasses several objectives. These include quantifying the abundance of Vibrio species and cyanobacteria within a selected shrimp farm, identifying the presence of two prominent Vibrio species known to pose risks to both humans and shrimps, and determining the specific genera of cyanobacteria present in that environment, followed by assessing potential Vibrio-cyanobacteria correlations with water quality parameters. It is hypothesized that the association between Vibrio species and cyanobacteria in shrimp farms is highly dependent on environmental factors such as water temperature, salinity, and pH. Methods such as most probable number (MPN), duplex-polymerase chain reaction (D-PCR), Sedgewick-Rafter cell counting, differential interference contrast (DIC) microscopy using an inverted microscope, and canonical correspondence analysis (CCA) were implemented in this study to achieve the objectives of this study.
Experimental Procedures
Water Sampling
Water sampling was commenced biweekly (August–December 2021) in two white-leg shrimp (Litopenaeus vannamei) in shrimp ponds (pond A and pond B) and effluent and influent water (Fig. 1) of Persatuan Nelayan Kawasan Satang Biru, Telaga Air, Kuching, Sarawak, Malaysia (1° 40′ 34.7″ N, 110° 12′ 10.8″ E). The sampling campaign was executed within the shrimps’ post-larvae stocking to harvesting time frame. The shrimp-rearing ponds have a surface area of 1 ha, a depth of 1.3 m, and a stocking density of 40 post-larvae (PL)/m2. A total of six (n = 6) samples from pond A, six (n = 6) samples from pond B, ten (n = 10) samples from effluent, and ten (n = 10) samples from influent were successfully collected, making up thirty-two (n = 32) samples. A plankton net was used to sift water from shrimp ponds to collect a concentrated amount of cyanobacteria inside 50-mL Falcon tubes. Environmental parameters such as water temperature, salinity, and pH were also recorded during the sampling activity—FisherbrandTM accumetTM AP125 Portable pH/Ion/mV/temperature meter kit (Thermo Fischer Scientific, USA) for the temperature and pH and refractometer (STAT, China). The samples were then transported to the laboratory under aseptic conditions within 2 h at room temperature.
Enrichment and Dilution of Vibrio Species
A total of 1 mL of the water samples were pipetted into 9 mL of Alkaline Peptone Water (APW) (HiMedia, India) to enrich Vibrio species in the samples. The cultures were then incubated overnight at 37 °C. Remaining water samples were treated with Lugol’s solution for the cyanobacteria counting step. After enrichment, 1 mL of culture broths was diluted up to 10−5. Each dilution was incubated overnight at 37 °C.
Most Probable Number (MPN)
MPN method from the US Food and Drug Administration’s Bacterial Analytical Manual (BAM) [23] was employed to enumerate the Vibrio species present in each water sample. This is done by making serial dilutions of the water samples and inoculating aliquots into broth media to detect bacterial growth. Firstly, 1 mL of the broths from the selected dilutions (10−2, 10−3, and 10−4) was inoculated into triplicates of 10 mL APW. The culture broths were then incubated for 24 h at 37 °C. The tubes that went turbid from each triplicate of MPN tubes from every dilution indicate growth for Vibrio species. The number of tubes that went turbid in each triplicate was recorded, and the result was referred to the MPN table [23]. In this study, we used a different approach compared to the traditional MPN table for presenting our results. Instead of using dilutions of 10−1, 10−2, and 10-3 like in a normal MPN table, the present study used dilutions of 10−2, 10−3, and 10−4. This modification allowed us to obtain accurate measurements and evaluate the microbial population more effectively. Therefore, each result for MPN per milliliter was multiplied by 10 to match the table configuration [24]. From each MPN tube that was incubated, a loopful of broth was streaked on the surface of thiosulfate-citrate-bile salts sucrose (TCBS) agar (HiMedia, India) and subsequently incubated overnight at 37 °C for Vibrio species confirmation.
DNA Extraction of Vibrio Species
The method was modified from Peng et al.’s [25] to fit the requirements of this study. Five hundred microliters of the culture broth was pipetted into a 1.5-mL microcentrifuge tube and centrifuged at 10,000 rpm for 5 min. The supernatant was discarded before adding 100 μL of deionized distilled water (ddH2O). The mixture was then boiled for 10 min and snap-cooled in ice for 5 min. Lastly, each tube was centrifuged for 10 min at 10,000 rpm. The clear solution is the product of the DNA extract.
Polymerase Chain Reaction (PCR)
PCR was done by adding exTEN PCR 2X Master Mix (Base Asia), 2 pairs of forward and reverse primers [26] as specified in Table 1, nuclease-free water, and DNA extracts from each sampling. In each PCR reaction, 12.5 μL of exTEN 2X PCR Master Mix (Base Asia) was used as the PCR reagent. The forward primers VP 1155272 F and VC C634002 F, as well as the reverse primers VP 1155272 R and VC C634002 R, were added at a volume of 0.6 μL each, with a concentration of 10 pmol/μL. Nuclease-free water was included at a volume of 1.1 μL. Additionally, 1.0 μL of DNA extract was added to the reaction mixture. The total volume of each PCR reaction was 17 μL.
The amplification of the DNA sequence was done by using a thermal cycler (Eppendorf Mastercycler Gradient) and with an initial denaturation step, performed at 94 °C for 6 min. Following this, denaturation takes place at 94 °C for 30 s. Annealing is conducted at 62 °C for 2 min, repeated for a total of 35 cycles. Subsequently, extension is carried out at 72 °C for 1 min and 50 s. Lastly, a final extension step occurs at 72 °C for 6 min.
Agarose Gel Electrophoresis (AGE)
The PCR products were loaded into 1.5% agarose gel and were charged with 80 V electric current for 1 h prior to being stained for 45 min with 0.1% ethidium bromide (EtBr). The bands formed were then observed by using a UV transilluminator before being photographed.
Quantification and Morphological Identification of Cyanobacteria
A total of 1 mL of water samples that were previously preserved with Lugol’s solution were pipetted by using a Pasteur pipette into Sedgewick-Rafter counting chambers exactly filling it while avoiding the formation of air bubbles. The water sample was then given about 5–10 min to settle down before being counted grid by grid using a compound microscope starting from left to right [27]. This process was repeated 3 times for each sample to gather the average count of the result. The number of cells per filament of the cyanobacteria was obtained by multiplying the total filaments counted with the average number of cells of the first 30 filaments [28]. The morphological identification of cyanobacteria was done by using an inverted microscope: Olympus FluoView 300 (Olympus Corporation, Japan). The microscope incorporates the differential interference contrast (DIC) filter in the process that can produce high-quality, pseudo-3D images of cyanobacteria (Scientifica, n.d.). The camera used to capture the images was called Infinity 3 (Teledyne Lumenera, Ottawa, Canada), while the software used to analyze the image was Infinity Analyze (Teledyne Lumenera, Ottawa, Canada).
Canonical Corresponding Analysis (CCA)
Data regarding the environmental variables obtained during the sampling activity and Vibrio and cyanobacteria populations was used to recognize any significant relationship between all the variables. The p-values are provided to indicate the statistical significance of Vibrio species and cyanobacteria. A p-value that is greater than 0.01 is considered not significant, while a p-value that is less than or equal to 0.01 indicates a significant relationship between the variables. The analysis was performed with PAST software version 4.03 (Palaeontological Association, Norway) which is freely available on the Internet at the time of writing.
Results
Environmental Parameters
Based on Table 2, the pond water pH ranges from 6.14 to 7.64, indicating that they were slightly acidic to neutral. While influent water was neutral, pond B was shown to develop the greatest amount of acidity on average. Minimal changes in water temperature for both ponds, effluent, and influent can be observed as they only slightly fluctuate. The salinity of ponds A and B both generally increased as the sampling process came to a close, contributing to the mean of 24. Evidently, the influent water had the lowest salinity compared to all sampling sites.
Growth Pattern and Identification of Vibrio Species
Pond A
The growth of Vibrio showed a notable rise during the second sampling and remained consistent until the conclusion of all samplings (Table 3). Vibrio species continued to be abundant until the end of the sampling cycle. In summary, PCR detection of V. cholerae and V. parahaemolyticus in pond A shows 72% positive detection of V. cholerae and 78% of V. parahaemolyticus (Fig. 2).
Duplex PCR result for the detection of V. parahaemolyticus and V. cholerae for pond A viewed in 1.5% agarose gel. M, 100 bp DNA ladder; 1, positive control of V. cholerae and V. parahaemolyticus; 2, negative control; 3, sampling 1; 4, sampling 2; 5–7, sampling 3; 8–9, sampling 4; 10–12, sampling 5; 13–15, sampling 6. Positive detection for V. parahaemolyticus is seen in lane 3 to 15, while positive detection for V. cholerae is seen in lane 3 to 14. V. cholerae bands appear to be faint when compared to V. parahaemolyticus
Pond B
The Vibrio species demonstrated rapid growth, reaching peak levels during each sampling period (Table 4). According to the findings obtained through the duplex-PCR technique, V. cholerae and V. parahaemolyticus were detected with a prevalence of 61% and 100%, respectively (Fig. 3).
Duplex PCR result for the detection of V. parahaemolyticus and V. cholerae for pond B viewed in 1.5% agarose gel. M, 100 bp DNA ladder; 1, positive control of V. cholerae and V. parahaemolyticus; 2, negative control; 3 and 4, sampling 1; 5, sampling 2; 6, sampling 3; 7 and 8, sampling 4; 9 and 10, sampling 5; 11 and 12, sampling 6. All samples show positive detection of V. parahaemolyticus, while the positive detection of V. cholerae is shown in lanes 4, 5, 7, 8, 9, 10, 11, and 12. V. cholerae bands appear to be faint when compared to V. parahaemolyticus with the exception of lane 5
Effluent
The initial effluent water sampling demonstrated minimal Vibrio proliferation, while the following samples indicated a rapid escalation in bacterial growth that remained consistent until the completion of the sampling period (Table 5). The detection of V. cholerae and V. parahaemolyticus gathered 90% of both species’ prevalence and can be observed in Fig. 4.
Duplex PCR result for the detection of V. parahaemolyticus and V. cholerae for effluent water viewed in 1.5% agarose gel. M, 100 bp DNA ladder; 1, positive control of V. cholerae and V. parahaemolyticus; 2, negative control; 3 and 4, sampling 2; 5, sampling 3; 6, sampling 4; 7, sampling 5; 8, sampling 6; 9, sampling 7; 10, sampling 8; 11, sampling 9; 12, sampling 10. Positive detection of V. parahaemolyticus can be seen in lanes 3, 5, 6, 7, 8, 9, 10, 11, and 12, same with V. cholerae. V. cholerae bands appear to be faint when compared to V. parahaemolyticus with the exception of lane 6
Influent
Vibrio growth can be seen off to a gradual increase during earlier sampling before the number persistently soared from the third sampling until the end (Table 6). As observed in Fig. 5, the duplex-PCR result of V. cholerae and V. parahaemolyticus showed successful detection of both species with 70% of V. cholerae and 90% of V. parahaemolyticus prevalence.
Duplex PCR result for the detection of V. parahaemolyticus and V. cholerae for influent water viewed in 1.5% agarose gel. M, 100 bp DNA ladder; 1, positive control of V. cholerae and V. parahaemolyticus; 2, negative control; 3, sampling 1; 4, sampling 2; 5, sampling 3; 6, sampling 4; 7, sampling 5; 8, sampling 6; 9, sampling 7; 10, sampling 8; 11, sampling 9; 12, sampling 10. Positive detection of V. parahaemolyticus can be seen on lanes 3 to 12, while positive detection of V. cholerae can be seen on lanes 3, 4, 7, 8, 9, 10, 11, and 12. V. cholerae bands are faint in comparison to V. parahaemolyticus with the exception of lane 4
Growth Pattern and Identification of Cyanobacteria
Figure 6 shows the growth pattern of cyanobacteria in every sampling site, across their sampling period. Pond A’s cyanobacterial growth curve gradually increased during the sample weeks. The 4th and 6th weeks, however, show a stagnant growth development. As for pond B, up until the 8th week, cyanobacterial growth indicated an overall increase. After that, there was a slight dip in the growth curve during week 10. The overall growth pattern for effluent displayed two peaks, one during the 4th week and the other in the 10th. However, it was clear that the peak of the 10th week was higher than the peak of the 4th week. The growth curve started to decline after the 10th week. The growth of cyanobacteria in the influent water can be observed to fluctuate, thus forming two peaks, which occurred during the 4th and 10th week. In comparison to the peak from the 4th week, the peak from the 10th week appeared to be higher. There was also a noticeable decline in growth during the 6th week, which appeared to be at the lowest point throughout the entire sampling period of this site.
A total of five members of the cyanobacteria genera were found residing in the shrimp farm water, namely, Chroococcus, Pseudanabaena, Phormidium, Oscillatoria, and Lyngbya. Based on cell counting with a Sedgewick-Rafter cell and morphological examination with an inverted microscope, it was evident that early samplings accumulate a greater diversity of cyanobacteria when compared to the conclusion of the sampling period, where Pseudanabaena genus can be seen dominating. The morphological assessment of the cyanobacterial species can be referred to in Table 7.
Canonical Correspondence Analysis (CCA)
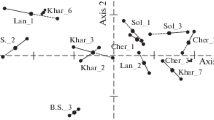
The association between Vibrio species and cyanobacteria in pond A, pond B, effluent, and influent water is shown in Fig. 7. Canonical correspondence analysis (CCA) was used to calculate the p-value for the correlation between both bacteria species. The water parameters were included to determine the distribution of Vibrio species and cyanobacteria according to abiotic factors. All p-values are insignificant (p > 0.01).
Figure 7A shows that the proliferation of cyanobacteria in pond A is predominantly associated with pH, salinity, and temperature as opposed to Vibrio species. The CCA for pond B (Fig. 7B) shows that Vibrio growth gravitates toward pH, while cyanobacteria growth gravitates toward salinity and temperature, although a minimal correlation between both bacteria can be seen. In effluent water (Fig. 7C), a connection can be seen between Vibrio and cyanobacteria, yet the growth of Vibrio is still primarily associated with pH levels rather than cyanobacteria, temperature, and salinity. Influent water shows no correlation between Vibrio species and cyanobacteria; however, cyanobacteria correlate with all environmental parameters (Fig. 7D).
In summary, both genera have a stronger relationship with environmental factors than they do with one another. The link between both genera is proven to be inconsistent with every sampling site and is minor. Cyanobacteria can be seen to prefer salinity at all sample locations, while Vibrio species were observed to prefer pH in pond B and effluent water. Compared to Vibrio species, cyanobacteria were seen to be more closely related to all water parameters.
Discussion
This study aimed to investigate the associations between Vibrio species and cyanobacteria in different sampling sites in a single shrimp farm, namely, pond A, pond B, effluent water, and influent water. CCA was used to examine the correlation between these bacteria species with respect to various abiotic factors such as pH, salinity, and temperature. The results showed that there is a stronger relationship between both genera and environmental parameters than between themselves. Cyanobacteria were found to prefer salinity at all sample locations, while Vibrio species were observed to have a preference for pH levels in pond B and effluent water. Overall, these findings underscore the complexity of the relationship between Vibrio species and cyanobacteria.
Water quality parameters act as a measure of the health and well-being of shrimp culture [29]. Maintaining ideal environmental conditions can ensure shrimps’ resilience to bacterial infections, to which they are particularly vulnerable [30]. In the sampling location where this study was conducted, pH, salinity, and temperature were monitored daily by the farmers. The mean pH for all the sampling sites was recorded to be along the line of 6.14 to 7.64. A moderate increase in the acidity of the shrimp farm water was observed as the sampling progressed toward the end. This might indicate the accumulation of decaying organic matter in shrimp farm waters [31]. This observation can be supported by the low pH displayed by pond A, pond B, and effluent that are in contact with shrimps but not from influent where the water comes into the shrimp farm from an outside source. Despite being slightly acidic, the level of pH remained in the normal range of 6.5 to 9.0 [32].
In previous studies, it has been observed that shrimps are highly sensitive to even slight variations in water temperature, which can have profound effects on their size [33]. Optimal shrimp yields have been reported at water temperatures ranging from 23.5 to 25.5 °C or between 30 and 31.5 °C [34]. Furthermore, the recommended range for maintaining ideal conditions during shrimp rearing is generally recognized as being between 28 and 32 °C [32]. In line with these findings, consistent water temperatures averaging from 30.8 to 31.5 °C were recorded in this study without significant fluctuations present. The water temperatures in the shrimp farm were within the optimal range for shrimp rearing, promoting favorable growth and yield. This result can be attributed to the consistent temperature characteristics of the coastal regions in Malaysia.
As for salinity, the optimum growing performance for shrimp was discovered between 20 and 30 ppt [35]. Likewise, the recorded salinity of the ponds (17.4 to 24 ppt) was well kept within the ideal range in all sampling sites. Shrimps can tolerate changes in salinity level as long as proper acclimation was done before stocking the post-larvae [36]. It is clear from the environmental parameters’ data that daily monitoring in shrimp farms is essential in ensuring the best possible water quality for the shrimps’ growth.
In addition to the parameters explored in this study, it is essential to acknowledge the role of another factor, the dissolved oxygen (DO) level, which also holds importance in ensuring the well-being of a shrimp farm ecosystem. The shrimp farmer involved in the research reported that the recorded range of DO on the farm was 5–8 mg/L, surpassing the optimum levels recommended by Robertson [37], which suggested 4 or 5 mg/L. The heightened DO levels observed in the shrimp farm can be attributed to the installation of an aeration system designed to ensure adequate DO levels [38]. The presence of phytoplankton, particularly through photosynthesis, also serves as the primary source of DO in water [39]. Additionally, factors that can have an effect on DO concentrations in water include temperature and salinity. When water temperature and salinity increase, oxygen solubility decreases [40, 41]. Recognizing the potential linkage between DO levels and the complex interactions among Vibrio species, cyanobacteria, and overall shrimp farm health, future research should address the study’s limitations by incorporating comprehensive dissolved oxygen data.
The interspecies relationship between the two genera has been proven to be an extremely complex discussion. In this research, a comparison was made between the growth of Vibrio bacteria and cyanobacteria in relation to different water parameters. Through the use of CCA, it was determined that pH, salinity, and temperature all play a role in affecting the growth of cyanobacteria; however, salinity had a more significant impact on their proliferation than other factors. This has also been proven by previous research [42]. Salinity can affect cyanobacteria in many ways such as their cell growth and their rate of photosynthesis [43]. Cyanobacteria’s tolerance toward salt might be due to the fact that they contain a vast regulatory mechanism that allows them to acclimatize in various salt concentrations [44]. Cyanobacteria’s high tolerance toward salt has been proven beneficial in the past for treating saline soil to improve rice crops [45]. In pond B and effluent water, Vibrio species were found to favor pH. However, other ponds (pond A and influent) indicate no major effect of pH toward the growth of Vibrio species. The variation in the result might indicate the optimum pH where they grow best [46].
Our finding is consistent with previous studies where a minimal interspecies correlation between Vibrio species and cyanobacteria was documented [47,48,49]. The differences in growth patterns between Vibrio species and cyanobacteria account for this phenomenon. When compared to the overall Vibrio species which population tends to be perpetually high, the growth curve of cyanobacteria displayed a clearer consistency. They have a structure resembling the lag phase, exponential or log phase, stationary phase, and death phase like normal bacterial growth. This is possible due to the naturally slow growth rate of cyanobacteria due to their ability to carry on complex mechanisms (such as the synthetization of amino acid and utilization of carbon dioxide for photosynthesis) causing the cells to prioritize cell function over reproduction [50]. In contrast to cyanobacteria, heterotrophic bacteria like Vibrio species use less energy to proliferate because they consume rather than produce their food, leading to their quicker and more rapid development profile [50]. This observation is also in line with a previous investigation conducted in the Pacific Northwest region of the USA in 2015, which reported minimal influence on Vibrio concentration due to the low abundance of cyanobacteria gathered [49]. Other aforementioned studies demonstrate that the interaction between the two genera is not a result of random events but rather influenced by various factors. The specific species involved [48], seasonal temperatures [47], and regional characteristics [51] play significant roles in determining this interspecies relationship. However, this finding should not completely rule out the compatibility of Vibrio proliferation with cyanobacterial blooms as 43–64% of the cyanobacterial bloom samples exhibited an association of viable but nonculturable forms of V. cholerae [52].
Throughout the sampling period, Vibrio species can be seen to either kick-start their growth slowly followed by a drastic increase in growth or maintain a constant high growth pattern above 1000 UFC/mL which is the maximum range of acceptable Vibrio population in healthy shrimp farm water [32]. V. cholerae was detected at rates of 72%, 61%, 90%, and 70% in pond A, pond B, effluent, and influent (respectively), while V. parahaemolyticus was identified at levels of 78%, 100%, 90%, and 90% using duplex-PCR methodology (Table 8). The detection of both Vibrio species is expected since they are naturally present in aquatic habitat ([53, 54]); however, the high concentrations are unforeseen due to the well-monitored water parameters. High concentrations of Vibrio in water are commonly viewed as a rather undesirable attribute as they can cause outbreaks of shrimp diseases [55]. Despite that, it is important to consider that not all Vibrio species in shrimp farms are pathogenic ([53, 54]); therefore, further testing for toxin genes was done to analyze the potential of the Vibrio species in causing infection. From the result, pronounced variation in the V. cholerae and V. parahaemolyticus prevalence can be explained by the halophilic profile of V. parahaemolyticus [7] which thrives in saline water better than V. cholerae. V. parahaemolyticus can also sustain a greater range of environmental differences such as pH and temperature in coastal water than V. cholerae [56]. Since the sampling was done in a coastal region, it is expected that V. parahaemolyticus population exceeds that of V. cholerae which explains the brighter bands displayed in duplex PCR.
Cyanobacteria may be very well distinguished from other bacteria based on the characteristics of their cell dimensions, shape, color, type of branching, sheath characteristics, and cell contents (summarized in Komárek and Anagnostidis [57,58,59,60]). They possess easily visible features that are easy to recognize, even at lower magnifications with light microscopy [61, 62]. Staining is rarely needed in cyanobacterial microscopy due to the presence of chloroplasts in their cells as natural blue-green pigments [63]. Some also contain red/brownish color due to a variety of pigments, like carotenoids and phycobiliproteins [63]. In the present study, it is apparent that the Pseudanabaena genus dominated the shrimp farm water as we approach the end of the sampling, significantly reducing the diversity of cyanobacteria. Aside from the reason that the Pseudanabaena genus can commonly be found inhabiting planktonic water, they also are highly tolerant toward disturbance and low light when compared to other cyanobacteria genera [64]. Chroococcus, Phormidium, Oscillatoria, and Lyngbya were among the other species that were discovered during prior samplings. In a nutshell, the study of cyanobacteria diversity in shrimp farms can lead to a better knowledge of their ecological roles, risk factors, and interactions with the shrimp farming system.
It can be concluded that environmental considerations such as pH, temperature, and salinity are more important factors in determining the growth of Vibrio species and cyanobacteria than their relationship with each other. It is suggested that reducing exposure to environmental stressors may be a more effective way to limit Vibrio and cyanobacterial growth than focusing on controlling one bacterium over another. In addition to water parameters, the monitoring of the dissolved oxygen level [65] and the population density of Vibrio species is suggested for the early prevention of shrimp diseases [55]. Additionally, several limitations of our study are the focus on a specific shrimp farming location which may limit the generalizability of our findings to other geographic regions or aquaculture systems, aside from the deficiency of DO level data. Further research is required to determine the precise mechanisms by which these bacteria interact with their environment and to develop more targeted interventions for managing them in aquaculture systems. A conclusive study involving a multidimensional landscape with a wider scope to multiple locations can link fragmentary knowledge regarding the relationship of both genera.
Conclusion
This study investigated the relationship between Vibrio species and cyanobacteria in a local shrimp farm. The results indicate that while there is a minor association between Vibrio species and cyanobacteria, other factors appear to have a stronger influence on the survival of Vibrio species. Specifically, pH levels were found to be more strongly correlated with the presence of Vibrio populations than cyanobacteria. Cyanobacteria, on the other hand, showed associations with factors such as pH, salinity, and temperature, with salinity playing a particularly influential role. Since other factors affecting the persistence of Vibrio species in shrimp farms may be present still, further studies should also explore other factors such as species-specific relationships, regional dynamics, and other multidimensional landscapes that may be affecting the persistence of Vibrio species in a shrimp farm. It is suggested that controlling water parameters can be more efficient in controlling vibriosis in shrimp farms compared to controlling cyanobacterial populations.
Dayang Najwa, A. B., Elexson, N., Dalene, L., & Teng, S. T. (2024). Vibrio Species and Cyanobacteria: Understanding their Association in Local Shrimp Farm using Canonical Correspondence Analysis (CCA). Microbial Ecology. 87(1). https://doi.org/10.1007/s00248-024-02356-5
Data Availability
All data generated or analyzed in this study are included in this published article.
References
Ismail MM, Abdullah AM (2013) Shrimp trade competitiveness of Malaysia and selected ASEAN countries. J Int Food Agribus Mark 25(sup1):98–115. https://doi.org/10.1080/08974438.2013.800009
Tawie S (2022) Sarawak aims to open up 7,000 hectares for shrimp farming, says state minister. Malay Mail Retrieved from https://www.malaymail.com/news/malaysia/2022/09/07/sarawak-aims-to-open-up-7000-hectares-for-shrimp-farming-says-state-minister/26969
Lembaga Kemajuan Ikan Malaysia Negeri Sarawak (LKIM). (2022). Retrieved form https://www.facebook.com/profile/100063528072203/search/?q=telaga%20&locale=ms_MY
Harris L, Owens L (1999) Production of exotoxins by two luminous Vibrio harveyi strains known to be primary pathogens of Penaeus monodon larvae. Dis Aquat Organ 38:11–22. https://doi.org/10.3354/dao038011
Prayitno SB, Latchford JW (1995) Experimental infections of crustaceans with luminous bacteria related to photobacterium and Vibrio: effect of salinity and pH on infectiosity. Aquaculture 132(1–2):105–112. https://doi.org/10.1016/0044-8486(94)00374-W
Duc PM, Hoa TT, Phuong NT, Bosma RH, Huỳnh VH, Tran NT (2015) Virus diseases risk-factors associated with shrimp farming practices in rice-shrimp and intensive culture systems in Mekong Delta Viet Nam. Int J Sci Res Publ 5:1–6 Retrieved from https://www.ijsrp.org/research-paper-0815/ijsrp-p4434.pdf
Cartwright EJ, Griffin PM (2012) 159—Other Vibrio species. In: Long SS (ed) Principles and practice of pediatric infectious diseasesfourth edn. Elsevier, pp 854–856.e1. https://doi.org/10.1016/B978-1-4377-2702-9.00161-6
Abd H, Valeru SP, Sami SM, Saeed A, Raychaudhuri S, Sandström G (2010) Interaction between Vibrio mimicus and Acanthamoeba castellanii. Environ Microbiol Rep 2(1):166–171. https://doi.org/10.1111/j.1758-2229.2009.00129.x
Austin B (2010) Vibrios as causal agents of zoonoses. Vet Microbiol 140(3-4):310–317. https://doi.org/10.1016/j.vetmic.2009.03.015
Venkateswaran K (1999) VIBRIO | Standard cultural methods and molecular detection techniques in foods. Encyclopedia Food Microbiol:2248–2258 Retrieved from https://www.sciencedirect.com/science/article/pii/B0122270703016652
Izumiya H, Matsumoto K, Yahiro S, Lee J, Morita M, Yamamoto S, Arakawa E, Ohnishi M (2011) Multiplex PCR assay for identification of three major pathogenic Vibrio spp., Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus. Mol Cell Probes 25(4):174–176. https://doi.org/10.1016/j.mcp.2011.04.004
Islam MS, Mahmuda S, Morshed MG, Bakht HBM, Khan MNH, Sack RB, Sack DA (2004) Role of cyanobacteria in the persistence of Vibrio cholerae O139 in saline microcosms. Can J Microbiol 50(2):127–131. https://doi.org/10.1139/w03-114
Mur LR, Skulberg OM et al (1999) Cyanobacteria in the environment. In: Chorus I, Bartram J (eds) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. London, E. and FN Spon (on Behalf of WHO), pp 15–40 Retrieved from https://www.who.int/publications/m/item/toxic-cyanobacteria-in-water%2D%2D-second-edition
Eiler A, Gonzalez-Rey C, Allen S, Bertilsson S (2007) Growth response of Vibrio cholerae and other Vibrio spp. to cyanobacterial dissolved organic matter and temperature in brackish water: cyanobacterial DOM, temperature and Vibrio growth. FEMS Microbiol Ecol 60(3):411–418. https://doi.org/10.1111/j.1574-6941.2007.00303.x
Dawes EA (1989) Growth and survival of bacteria. In: Poindexter JS, Leadbetter ER (eds) Bacteria in Nature, vol 3. Springer, Boston, MA. https://doi.org/10.1007/978-1-4613-0803-4_2
Rojas V, Rivas L, Cárdenas C, Guzmán F (2020) Cyanobacteria and eukaryotic microalgae as emerging sources of antibacterial peptides. Molecules 25:24. https://doi.org/10.3390/molecules25245804
King K, Bramucci AR, Labbate M, Raina J-B, Seymour JR (2022) Heterogeneous growth enhancement of Vibrio cholerae in the presence of different phytoplankton species. Appl Environ Microbiol 88(17):e01158–e01122. https://doi.org/10.1128/aem.01158-22
Larsen JP (1984) Vibrio anguillarum: influence of temperature, pH, NaCl concentration and incubation time on growth. J Appl Microbiol 57(2):237–246. https://doi.org/10.1111/j.1365-2672.1984.tb01388.x
Sheikh HI, Najiah M, Fadhlina A, Laith AA, Nor MM, Jalal KCA, Kasan NA (2022) Temperature upshift mostly but not always enhances the growth of Vibrio species: a systematic review. Front Mar Sci 9:959830. https://doi.org/10.3389/fmars.2022.959830
Chen Y-H, He J (2019) Effects of environmental stress on shrimp innate immunity and white spot syndrome virus infection. Fish Shellfish Immunol 84:744–755. https://doi.org/10.1016/j.fsi.2018.10.069
Maicá PF, Borba MR, Martins TG, Wasielesky Junior W (2014) Effect of salinity on performance and body composition of Pacific white shrimp juveniles reared in a super-intensive system. Rev Bras Zootec 43(7):343–350. https://doi.org/10.1590/s1516-35982014000700001
Kopprio GA, Neogi SB, Rashid H, Alonso C, Yamasaki S, Koch BP, Gärdes A, Lara RJ (2020) Vibrio and bacterial communities across a pollution gradient in the Bay of Bengal: unraveling their biogeochemical drivers. Front Microbiol 11:594. https://doi.org/10.3389/fmicb.2020.00594
BAM Appendix 2: Most probable number from serial dilutions. (2020). Retrieved from https://www.fda.gov/food/laboratory-methods-food/bam-appendix-2-most-probable-number-serial-dilutions
Sutton S (2010) The most probable number method and its uses in enumeration, qualification, and validation. J Valid Technol 16(3):35–38 Retrieved from https://www.semanticscholar.org/paper/The-Most-Probable-Number-Method-and-Its-Uses-in-and-Sutton/2185acb4e79908a9e68e4b80f5b484398042dfff
Peng X, Yu K-Q, Deng G-H, Jiang Y-X, Wang Y, Zhang G-X, Zhou H-W (2013) Comparison of direct boiling method with commercial kits for extracting fecal microbiome DNA by Illumina sequencing of 16S rRNA tags. J Microbiol Methods 95(3):455–462. https://doi.org/10.1016/j.mimet.2013.07.015
Kim H-J, Ryu J-O, Lee S-Y, Kim E-S, Kim H-Y (2015) Multiplex PCR for detection of the Vibrio genus and five pathogenic Vibrio species with primer sets designed using comparative genomics. BMC Microbiol 15(1):239. https://doi.org/10.1186/s12866-015-0577-3
Watzin MC, Miller EB, Shambaugh AD, Kreider MA (2006) Application of the WHO alert level framework to cyanobacterial monitoring of Lake Champlain, Vermont. Environ Toxicol 21(3):278–288. https://doi.org/10.1002/tox.20181
Chorus I, Bartram J (eds) (1999) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring, and management. E & FN Spon Retrieved from https://www.who.int/publications/m/item/toxic-cyanobacteria-in-water%2D%2D-second-edition
Carbajal-Hernández JJ, Sánchez-Fernández LP, Villa-Vargas LA, Carrasco-Ochoa JA, Martínez-Trinidad JF (2013) Water quality assessment in shrimp culture using an analytical hierarchical process. Ecol Indic 29:148–158. https://doi.org/10.1016/j.ecolind.2012.12.017
Cheng W, Chen S-M, Wang F-I, Hsu P-I, Liu C-H, Chen J-C (2003) Effects of temperature, pH, salinity and ammonia on the phagocytic activity and clearance efficiency of giant freshwater prawn Macrobrachium rosenbergii to Lactococcus garvieae. Aquaculture 219(1–4):111–121. https://doi.org/10.1016/S0044-8486(03)00017-6
Zhang, H. (2017). Cause and effects of soil acidity. 2. Retrieved from https://extension.okstate.edu/fact-sheets/cause-and-effects-of-soil-acidity.html
Carbajal-Hernández JJ, Sánchez-Fernández LP, Carrasco-Ochoa JA, Martínez-Trinidad JF (2012) Immediate water quality assessment in shrimp culture using fuzzy inference systems. Expert Syst Appl 39(12):10571–10582. https://doi.org/10.1016/j.eswa.2012.02.141
Wyban J, Walsh W, Godin D (1995) Temperature effects on growth, feeding rate and feed conversion of the Pacific white shrimp (Penaeus vannamei). Aquaculture 138:267–279. https://doi.org/10.1016/0044-8486(95)00032-1
Abdelrahman H, Abebe A, Boyd C (2019) Influence of variation in water temperature on survival, growth and yield of Pacific white shrimp Litopenaeus vannamei in inland ponds for low-salinity culture. Aquacult Res 50(2):658–672. https://doi.org/10.1111/are.13943
Gao W, Tian L, Huang T, Yao M, Hu W, Xu Q (2016) Effect of salinity on the growth performance, osmolarity and metabolism-related gene expression in white shrimp Litopenaeus vannamei. Aquac Rep 4:125–129. https://doi.org/10.1016/j.aqrep.2016.09.001
Rahi ML, Azad KN, Tabassum M, Irin HH, Hossain KS, Aziz D, Moshtaghi A, Hurwood DA (2021) Effects of salinity on physiological, biochemical and gene expression parameters of black tiger shrimp (Penaeus monodon): potential for farming in low-salinity environments. Biology 10(12):1220. https://doi.org/10.3390/biology10121220
Robertson CE (2006) Australian prawn farming manual: health management for profit. Manual. The State of Queensland, Department of Primary Industries and Fisheries, Brisbane http://era.daf.qld.gov.au/id/eprint/2055/
Browdy CL, Bratford D, Stokes AD, Mcintosh RP (2001) Perspectives on the application of closed shrimp culture systems. Environ Sci https://www.researchgate.net/publication/250309306_Perspectives_on_the_application_of_closed_shrimp_culture_systems
Liqoarobby R, Sunardi S, Y., & Fadilah, K. (2021) Aqueous systems of dissolved oxygen in reservoir. E3S Web Conf 249(2021):03015. https://doi.org/10.1051/e3sconf/202124903015v
Lange R, Staaland H, Mostad A (1972) The effect of salinity and temperature on solubility of oxygen and respiratory rate in oxygen-dependent marine invertebrates. J Exp Mar Biol Ecol 9(3):217–229. https://doi.org/10.1016/0022-0981(72)90034-2
Ali B, Anushka, Mishra A (2002) Effects of dissolved oxygen concentration on freshwater fish: a review. Int J Fish Aquat 10(4):113–127. https://doi.org/10.22271/fish.2022.v10.i4b.2693
Silveira SB, Odebrecht C (2019) Effects of salinity and temperature on the growth, toxin production, and akinete germination of the cyanobacterium Nodularia spumigena. Front Mar Sci 6:339 https://www.frontiersin.org/articles/10.3389/fmars.2019.00339
Moisander PH, McClinton E, Paerl HW (2002) Salinity effects on growth, photosynthetic parameters, and nitrogenase activity in estuarine planktonic cyanobacteria. Microb Ecol 43(4):432–442. https://doi.org/10.1007/s00248-001-1044-2
Pade N, Hagemann M (2014) Salt acclimation of cyanobacteria and their application in biotechnology. Life 5(1):25–49. https://doi.org/10.3390/life5010025
Jan Z, Ali S, Sultan T, Khan MJ, Shah Z, Khan F (2018) Impact of different strains of cyanobacteria on rice crop growth and nutrients uptake under saline soil condition. Sarhad J Agric 34(2):450–458. https://doi.org/10.17582/journal.sja/2018/34.2.450.458
Keenleyside, W. (2019). 9.5 The effects of pH on microbial growth. https://ecampusontario.pressbooks.pub/microbio/chapter/the-effects-of-ph-on-microbial-growth/
Greenfield DI, Gooch Moore J, Stewart JR, Hilborn ED, George BJ, Li Q, Dickerson J, Keppler CK, Sandifer PA (2017) Temporal and environmental factors driving Vibrio vulnificus and V. parahaemolyticus populations and their associations with harmful algal blooms in South Carolina detention ponds and receiving tidal creeks. GeoHealth 1(9):306–317. https://doi.org/10.1002/2017GH000094
Julie D, Solen L, Antoine V, Jaufrey C, Annick D, Dominique H-H (2010) Ecology of pathogenic and non-pathogenic Vibrio parahaemolyticus on the French Atlantic coast. Effects of temperature, salinity, turbidity and chlorophyll A: ecology of Vibrio parahaemolyticus in France. Environ Microbiol 12(4):929–937. https://doi.org/10.1111/j.1462-2920.2009.02136.x
Paranjpye RN, Nilsson WB, Liermann M, Hilborn ED, George BJ, Li Q, Bill BD, Trainer VL, Strom MS, Sandifer PA (2015) Environmental influences on the seasonal distribution of Vibrio parahaemolyticus in the Pacific Northwest of the USA. FEMS Microbiol Ecol 91(12):fiv121. https://doi.org/10.1093/femsec/fiv121
Burnap RL (2015) Systems and photosystems: cellular limits of autotrophic productivity in cyanobacteria. Front Bioeng Biotechnol 3:1. https://doi.org/10.3389/fbioe.2015.00001
Turner JW, Good B, Cole D, Lipp EK (2009) Plankton composition and environmental factors contribute to Vibrio seasonality. ISME J 3(9):1082–1092. https://doi.org/10.1038/ismej.2009.50
Chaturvedi P, Agrawal M, Bagchi SN (2015) Microcystin-producing and non-producing cyanobacterial blooms collected from the Central India harbor potentially pathogenic Vibrio cholerae. Ecotoxicol Environ Saf 115:67–74. https://doi.org/10.1016/j.ecoenv.2015.02.001
Garibay-Valdez E, Martínez-Córdova LR, López-Torres MA, Almendariz-Tapia FJ, Martínez-Porchas M, Calderón K (2020) The implication of metabolically active Vibrio spp. In the digestive tract of Litopenaeus vannamei for its post-larval development. Sci Rep 10(1):1. https://doi.org/10.1038/s41598-020-68222-9
Zoqratt MZHM, Eng WWH, Thai BT, Austin CM, Gan HM (2018) Microbiome analysis of Pacific white shrimp gut and rearing water from Malaysia and Vietnam: implications for aquaculture research and management. PeerJ 6:e5826. https://doi.org/10.7717/peerj.5826
de Souza Valente C, Wan AHL (2021) Vibrio and major commercially important vibriosis diseases in decapod crustaceans. J Invertebr Pathol 181:107527. https://doi.org/10.1016/j.jip.2020.107527
Prasanthan V, Purayath U, Sarathkumar, & Ouseph, P. P. (2011) Influence of abiotic environmental factors on the abundance and distribution of Vibrio species in coastal waters of Kerala, India. Indian J Mar Sci 40:587–592 Retrieved from https://www.researchgate.net/publication/286382075_Influence_of_abiotic_environmental_factors_on_the_abundance_and_distribution_of_Vibrio_species_in_coastal_waters_of_Kerala_India
Dvorak P, Casamatta D, Hasler P, Jahodářová E, Norwich A, Poulíčková A (2017) Diversity of the cyanobacteria. Modern Topics in the Phototrophic Prokaryotes: Environmental and Applied Aspects, vol 3–46. https://doi.org/10.1007/978-3-319-46261-5_1
Komárek J (2013) Cyanoprokaryota. 3. Heterocytous genera. In: Büdel B, Gärtner G, Krienitz L, Schagerl M (eds) Süswasserflora von Mitteleuropa/Freshwater flora of Central Europe. Springer Spektrum, Berlin/Heidelberg/Germany, pp 1–1130. https://doi.org/10.1007/978-3-319-46261-5_1
Komárek J, Anagnostidis K (2005) Cyanoprokaryota: Oscillatoriales in Süßwasserflora von Mitteleuropa. Spektrum Akademischer Verlag 19(2):1–759
Komárek J, Anagnostidis K (1998) Cyanoprokaryota: Chroococcales. In: Ettl H, Gärtner G, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, vol 19, no 1. Gustav Fischer, Jena-Stuttgart-Lübeck-Ulm, p 548
Albrecht M, Pröschold T, Schumann R (2017) Identification of cyanobacteria in a eutrophic coastal lagoon on the Southern Baltic Coast. Front Microbiol 8:923. https://doi.org/10.3389/fmicb.2017.00923
Yu G, Zhu M, Youxin C, Li R (2015) Polyphasic characterization of four species of Pseudanabaena (Oscillatoriales, Cyanobacteria) from China. ResearchGate; Magnolia Press https://www.researchgate.net/publication/282279865_Polyphasic_characterization_of_four_species_of_Pseudanabaena_Oscillatoriales_Cyanobacteria_from_China_and_insights_into_polyphyletic_divergence_within_the_Pseudanabaena_genus
Saini DK, Pabbi S, Shukla P (2018) Cyanobacterial pigments: perspectives and biotechnological approaches. Food Chem Toxicol 120:616–624. https://doi.org/10.1016/j.fct.2018.08.002
Nienaber MA, Steinitz-Kannan M (2018) A guide to cyanobacteria: identification and impact. University Press of Kentucky Retrieved from https://www.perlego.com/book/874533/a-guide-to-cyanobacteria-identification-and-impact-pdf
Dien LD, Hiep LH, Faggotter SJ, Chen C, Sammut J, Burford MA (2019) Factors driving low oxygen conditions in integrated rice-shrimp ponds. Aquaculture 512:734315. https://doi.org/10.1016/j.aquaculture.2019.734315
Acknowledgements
This work forms parts of the master project of Dayang Najwa binti Awg Baki in collaboration with Lembaga Kemajuan Ikan Malaysia (LKIM).
Funding
The project was funded by the Ministry of Higher Education Malaysia through the Fundamental Research Grant Scheme (FRGS) (FRGS/1/2019/STG05/UNIMAS/03/2) Universiti Malaysia Sarawak, Kota Samarahan, Kuching Sarawak.
Author information
Authors and Affiliations
Contributions
Dayang Najwa Binti Awg Baki: Formal analysis (Lead); Methodology (Lead); Resources (Lead); Software (Lead); Visualization (Lead); Writing – original draft (Lead); Writing – review & editing (Lead); Elexson Nillian: Funding acquisition (Lead); Investigation (Supporting); Supervision (Lead); Validation (Equal); Writing – review & editing (Supporting); Dalene Lesen: Funding acquisition (Supporting); Writing – review & editing (Supporting); Teng Sing Tung: Conceptualization (Supporting); Formal analysis (Supporting); Methodology (Supporting); Software (Supporting); Supervision (Supporting); Validation (Supporting); Visualization (Supporting).
Corresponding author
Ethics declarations
Ethics Statement
None required.
Conflict of Interest
The authors declare no competing interests.
Supplementary information
ESM 1
(DOCX 28 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dayang Najwa, A.B., Elexson, N., Dalene, L. et al. Vibrio Species and Cyanobacteria: Understanding Their Association in Local Shrimp Farm Using Canonical Correspondence Analysis (CCA). Microb Ecol 87, 51 (2024). https://doi.org/10.1007/s00248-024-02356-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00248-024-02356-5