Abstract
In the 2019–2020 summer, wildfires decimated the Australian bush environment and impacted wildlife species, including koalas (Phascolarctos cinereus) and grey headed flying fox pups (Pteropid bats, Pteropus poliocephalus). Consequently, hundreds of koalas and thousands of bat pups entered wildlife hospitals with fire-related injuries/illness, where some individuals received antimicrobial therapy. This study investigated the dynamics of antimicrobial resistance (AMR) in pre-fire, fire-affected and post-fire koalas and Pteropid bat pups. PCR and DNA sequencing were used to screen DNA samples extracted from faeces (koalas and bats) and cloacal swabs (koalas) for class 1 integrons, a genetic determinant of AMR, and to identify integron-associated antibiotic resistance genes. Class 1 integrons were detected in 25.5% of koalas (68 of 267) and 59.4% of bats (92 of 155). Integrons contained genes conferring resistance to aminoglycosides, trimethoprim and beta-lactams. Samples were also screened for blaTEM (beta-lactam) resistance genes, which were detected in 2.6% of koalas (7 of 267) and 25.2% of bats (39 of 155). Integron occurrence was significantly higher in fire-affected koalas in-care compared to wild pre-fire koalas (P < 0.0001). Integron and blaTEM occurrence were not significantly different in fire-affected bats compared to pre-fire bats (P > 0.05), however, their occurrence was significantly higher in fire-affected bats in-care compared to wild fire-affected bats (P < 0.0001 and P = 0.0488 respectively). The observed shifts of AMR dynamics in wildfire-impacted species flags the need for judicious antibiotic use when treating fire-affected wildlife to minimise unwanted selective pressure and negative treatment outcomes associated with carriage of resistance genes and antibiotic resistant bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimicrobial resistance (AMR) is now widespread in diverse wildlife species [1, 2]. The past decade has seen a rapid increase in reports of antimicrobial resistant bacteria in wildlife [3], including in Australian wild mammals and birds [4,5,6,7,8,9,10]. The carriage of antimicrobial resistant bacteria by wildlife poses a risk to their health when an individual requires antibiotic treatment, potentially leading to negative treatment outcomes associated with antibiotic resistant bacterial pathogens [11,12,13]. For some wildlife species, the carriage of AMR is higher is animals which have entered veterinary hospitals and rehabilitation environments compared to their free-living counterparts [8, 14, 15]. A higher prevalence of AMR in wildlife in captive settings (both wild animals in-care and wildlife in zoological collections) has been associated with multiple factors including, the administration of antibiotics, increased exposure to antimicrobial resistant bacteria and increased transmission of resistant bacteria between co-housed individuals in-care [8, 15,16,17].
The rise of AMR can, in part, be attributed to the rapid spread of mobile genetic elements (MGE), such as plasmids and transposons, which carry diverse antimicrobial resistance genes (ARG) [18]. MGE facilitate the horizontal transfer of ARG and associated genetic mechanisms between bacteria of different strains and species [18]. The class 1 integron is a genetic mechanism which has played a significant role in the dissemination of AMR [19], particularly in Gram-negative bacterial pathogens [20]. The class 1 integron has key components that enable it to capture, integrate and express genes that confer resistance to a diverse range of antibiotics [21]. Class 1 integrons are comprised of an integrase gene (intl1), a promoter (Pc), a variable number of gene cassettes encoding ARGs, which form a gene cassette array, and typically a 3’-conserved segment (qacEdelta) [21]. Alternatively, class 1 integrons may have an IS26 transposase in place of the typical qacEdelta [22].
The class 1 integron has been proposed as an indicator of anthropogenic DNA pollution in the environment and subsequently, in wildlife [23]. Wildlife in habitats located within close proximity to humans and/or domestic animals typically have a higher prevalence of class 1 integrons compared to wildlife living in remote natural environments [24, 25]. In Australian wildlife, the class 1 integron has been detected in diverse species, including the grey-headed flying fox (Pteropus poliocephalus) [14], the little penguin (Eudyptula minor) [26], the brush-tailed rock-wallaby (Petrogale penicillate) [25], the Australian sea lion (Neophoca cinerea) and the Australian fur seal (Arctocephalus pusillus doriferus) [4], indicating that antibiotic resistant bacteria are well integrated in wildlife gut microbiomes.
Beta-lactam antibiotics, including penicillin, amoxicillin and cephalosporins, are the most abundant antibiotic class administered to humans [27], domestic animals [28], and also commonly used in wildlife medicine [29]. Resistance to beta-lactam antibiotics is predominantly associated with the production of beta-lactamases, of which, blaTEM is one of the most common found in Gram-negative bacteria [30]. Along with class 1 integrons, the blaTEM gene can be used as an indicator of anthropogenic AMR in the environment [31]. The blaTEM gene has been detected in Australian wildlife species, including the grey-headed flying fox (P. poliocephalus) [6, 7] and Australian silver gulls (Chroicocephalus novaehollandiae) [5].
In the summer of 2019–2020, catastrophic wildfires extended across south-eastern Australia, resulting in the destruction of almost 12.6 million hectares [32, 33] and the loss of hundreds of millions of native animals [33, 34]. In addition to the millions of native animals directly lost or impacted by the wildfires (primary fire-affected wildlife), millions of animals were also displaced, faced habitat loss, food and water shortages, or acquired other injuries (secondary fire-affected wildlife) [34]. Native wildlife species impacted by the 2019–2020 fires included mammals (inclusive of marsupials and monotremes), reptiles, frogs and birds [34]. The 2019–2020 wildfires in Australia are part of a wider global fire threat for biodiversity and wildlife arising from climate change [35, 36].
The koala (Phascolarctos cinereus), an iconic Australian arboreal marsupial, was one of the most heavily impacted species in the 2019–2020 wildfires [34, 37,38,39,40]. In South Australia, approximately 280,000 hectares were burnt and an estimated 40,000 to 50,000 koalas died [41], with the vast majority of losses occurring on Kangaroo Island, South Australia [34]. Although koalas in South Australia are not listed as a threatened species, unlike the endangered eastern populations [42], it is estimated that up to 85% of the Kangaroo Island koala population was lost in the 2019–2020 wildfires [34]. Several hundred primary and secondary fire-affected koalas which survived the fires, were taken into care on Kangaroo Island and on the South Australian mainland, and treated for burns, injuries, disease, starvation and/or dehydration [34, 37].
The grey headed flying fox (P. poliocephalus) is an endemic Pteropid bat species found across eastern Australia [43]. They roost in trees located in both urban and forested areas, and typically form colonies containing up to 50,000 individuals [43]. Grey headed flying foxes (hereon after bat) are highly mobile, flying long distances to forage each night (> 50 km) and travelling hundreds of kilometres between colonies [44]. Each year, bat pups are born between October and December, and spend the first month of life carried by their mothers [45]. After this time, pups remain in the bat roost at night, while their mothers fly out to forage, until they are old enough to forage for themselves (3–4 months) [45, 46]. Grey headed flying foxes are listed as vulnerable to extinction on the IUCN Red List [47], and prior to the 2019–2020 wildfires they were already being negatively impacted by habitat loss, drought, extreme heat events and disease [43]. During the 2019–2020 wildfires, an unknown number of bats were killed or directly impacted by wildfires (i.e. primary fire-affected bats) [34, 48]. A large number, estimated to be > 70,000 bats, were lost due to fire-related impacts including pup abandonment [49], habitat loss and food shortages [34], termed secondary fire-affected bats. During the 2019–2020 wildfires, over 2,500 bat pups and adults were rescued and taken into care until they were deemed suitable for release back into wild colonies [49].
This study aimed to investigate the impact of fire on the dynamics of AMR determinants in fire-affected wildlife. The occurrence and diversity of class 1 integrons and blaTEM genes in DNA samples from pre-fire, fire-affected and post-fire koalas (faecal and cloacal samples) and bat pups (faecal samples) were assessed. Additionally, the study aimed to evaluate the association of class 1 integron and blaTEM carriage with fire-season status, entry into care and antibiotic administration.
Methods
Koala Faecal Sampling and DNA Extraction
Faecal samples (n = 190) were opportunistically collected from wild-caught koalas at three sites (Cleland Conservation Park, Morialta Conservation Park and Belair National Park) located within the Mount Lofty Ranges, South Australia, and provided for this study (Supplementary Data 1). These three sites (Cleland Conservation Park, Morialta Conservation Park and Belair National Park) are unfenced wild koala habitat located 10–12 km from Adelaide city centre. Koalas in Cleland and Morialta Conservation Parks were sampled once (April 2018) and koalas in the Belair National Park were sampled twice (April 2018 and October 2022) (Supplementary Data 1). Relative to the 2019–2020 wildfires, the 190 samples were divided into pre-fire samples (n = 91, collected April 2018) and post-fire samples (n = 99, collected October 2022) (Supplementary Data 1). Faecal samples were obtained by either rectal swab (n = 113) (COPAN, Brescia, Italy) or from faecal pellets (n = 77) (Supplementary Data 1). Samples were stored at 4˚C until transferred to the laboratory, then frozen at -30˚C until processing. DNA was extracted from faecal samples using the ISOLATE II Fecal DNA Kit (Bioline, London, UK). Metadata was provided for all individual koalas (Supplementary Data 1).
Female Koala Cloacal Swab DNA Samples
A subset of samples were provided as DNA extractions that were derived from female koala cloacal swab samples (n = 81) collected from koalas on Kangaroo Island, between 2014 and 2017 (represented pre-fire samples) and during the 2019–2020 wildfires (represented fire-affected samples) (Supplementary Data 1). The pre-fire DNA samples (n = 49) were from wild female koalas caught as part of a koala sterilisation program run by the South Australian Department for Environment and Water (DEW) between 2014 and 2017 [50] (Supplementary Data 1). The fire-affected samples (n = 32) were obtained from previously wild female koalas that entered into veterinary care during the 2019–2020 wildfires [37] (Supplementary Data 1). All samples were stored at -80˚C or -30˚C. Metadata was provided for all individual koalas (Supplementary Data 1).
Grey Headed Flying Fox Faecal Sampling and DNA Extraction
Bat faecal samples (n = 155) were collected from pups at three locations in New South Wales (Sydney, Shoalhaven and Bega) and one location in South Australia (Adelaide) (Supplementary Data 1). Bat pups in Shoalhaven and Bega were sampled once (during one birthing season), and pups in Adelaide and Sydney were sampled twice (during two different birthing seasons) (Supplementary Data 1). Relative to the 2019–2020 wildfires, the 155 samples were classified as pre-fire samples (n = 77, collected October 2018 to April 2019), fire-affected samples (n = 48, collected November 2019 to February 2020), and post-fire samples (n = 30, collected January 2021) (Supplementary Data 1). Most of the sampled bat pups were in-care (118 of 155) and the remaining were wild (37 of 155) (Supplementary Data 1). The FecalSwab™ system (COPAN, Brescia, Italy) was used to collect all faecal samples, which included faecal deposits from 116 live bat pups and faecal material from the intestines of 39 deceased pups that had been stored at -20˚C until necropsy. Metadata is provided for all individual bat pups (Supplementary Data 1). FaecalSwab samples were frozen at -30˚C until processing. DNA was extracted from FecalSwab media using the ISOLATE II Fecal DNA Kit (Bioline, London, UK) and stored at -30˚C.
16 S rRNA PCR Screening of DNA Samples
All DNA samples (n = 426) underwent a 16 S rRNA PCR to confirm DNA competency prior to inclusion in screening for integrons and blaTEM genes. The 16 S rRNA PCR was performed using the universal eubacterial primers f27 and r1492 [51] as previously described [52]. Only samples which amplified in the 16 S rRNA PCR, and deemed PCR-competent, were utilised in this study (Supplementary Data 1).
PCR Screening for Class 1 Integrons
All faecal and cloacal swab DNA samples deemed PCR-competent were then screened for the presence of class 1 integrons. Primers HS463a and HS464 [53] were used to target the class 1 integron integrase gene (intI1) using using GoTaq® Colourless Master Mix (Promega, Madison, WI, USA) and PCR conditions as previously described [14]. All PCRs included an intI1 positive control sample (E. coli strain KC2) [19], and a negative control (PCR-grade H2O), and were visualised using gel electrophoresis.
PCR Screening for Gene Cassette Arrays
Samples identified as intI1 positive were then screened using two further PCRs to detect gene cassette arrays and identify ARGs. Two primers sets were used to target gene cassette arrays with either the conserved 3’ terminus (primers HS458 and HS459) [54] or the alternate IS26 transposase 3’ terminus (primers HS458 and JL-D2) [22]. Both the HS458/HS459 and HS458/JL-D2 PCRs used GoTaq® Colourless Master Mix (Promega, Madison, WI, USA) and the same PCR conditions, as previously described for HS458/HS459 [14]. All PCRs included a positive control sample; E. coli strain KC2 as the HS458/459 positive control [19] or E. coli strain FF993W as the HS458/JL-D2 positive control [6], and a negative control (PCR-grade H2O), and PCR products were visualised using gel electrophoresis.
Where a single amplicon was present, PCR products were purified using the MinElute PCR Purification Kit (Qiagen, Hilden, Germany). Purified PCR products underwent DNA sequencing at The Ramaciotti Centre for Genomics (University of New South Wales, Sydney, Australia) using Big Dye Terminator chemistry version 3.1 and ABI 3730/3730 × 1 Capillary Sequencers (Applied Biosystems, Foster City, CA, USA). DNA sequences were analysed using Geneious Prime software (versions R11 to 2023.1.2; Biomatters Limited, Auckland, New Zealand) and gene cassette arrays were identified using BLASTN searches (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and ARG variants confirmed using ResFinder 4.1 (available at http://www.genomicepidemiology.org/services/) [55]. Where multiple PCR amplicons were present, bright gel bands were excised and purified using the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) and then sequenced, analysed and ARGs identified as described above for single amplicon HS458/HS459 PCR products. Where gene cassette arrays were not observed in IntI1 positive samples, HS463a/HS464 PCR products were purified, sequenced, and analysed as described above for HS458/HS459 PCR products. DNA sequences were confirmed as intI1 genes using BLASTN searches (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
PCR Screening for bla TEM Genes
All faecal and cloacal swab DNA samples deemed to be PCR-competent were also screened for the presence of blaTEM genes using the primers TEM-C 5’TCGGGGAAATGTGCGCG3’ and TEM-D 5’TGCTTAATCAGTGAGGCACC3’ [56].
The blaTEM PCRs used TopTaq DNA Polymerase (Qiagen, Hilden, Germany) and cycling conditions of 94 °C 3 min; 35 cycles of 94 °C 30 s, 50 °C 20 s, 72 °C 2 min; 72 °C 5 min. All PCRs included a blaTEM-1B positive control sample (E. coli strain FF993W) [6] and a negative control (PCR-grade H2O), and were visualised using gel electrophoresis. The blaTEM PCR products were purified, sequenced, analysed and blaTEM genes confirmed as described above for intI1 PCR products. To identify blaTEM gene variants, representative sequences were uploaded to ResFinder 4.1 (available at http://www.genomicepidemiology.org/services/ and accessed in May 2023) [55].
Class 1 Integron and bla TEM Sequence Annotation and Accessions
Representative sequences for intI1, gene cassette arrays and blaTEM genes were manually annotated in Geneious Prime software (version 2023.1.2; Biomatters Limited, Auckland, New Zealand) using reference sequences identified in BLASTN searches (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and uploaded to GenBank under accession numbers OR095837 - OR095843 and OR095845 - OR095856 (Supplementary Data 1).
Statistical Analysis
Fisher’s exact test (two-tailed) was used to evaluate any statistical difference in the occurrence of intI1 and blaTEM genes in paired samplings collected from the same locations at different time points (i.e., pre-fire/fire-affected/post-fire), from different environments (i.e., wild and in-care), and with or without antibiotic administration. Calculations were performed using GraphPad (available at https://www.graphpad.com), with P < 0.05 indicating statistical significance.
Results
Detection of Class 1 Integrons in Koalas and Bats
The intI1 gene, signalling the presence of the class 1 integron, was detected in 25.5% of all PCR-competent koala DNA samples (68 of 267), and ranged from 0.0 to 46.9% across the six sampling events (Table 1). In Mount Lofty Ranges koalas, integron frequency ranged from 6.9 to 43.6% and from 0.0 to 46.9% in Kangaroo Island koalas (Table 1). In bat pups, the intI1 gene was detected in 59.4% of all PCR-competent DNA samples (92 of 155) and ranged from 23.3 to 100% across the six sampling events (Table 1).
A total of 46 class 1 integrons containing ARGs were identified in koalas and bats, which were differentiated into integrons with the conserved 3’ terminus (qacEdelta; n = 27) and those with the alternate IS26 transposase 3’ terminus (IS26; n = 19) (Table 1). Ten distinct types of class 1 integrons carrying ARGs were identified in both wildlife hosts, with four types detected in koala DNA samples and eight types in bat DNA samples (Table 1). The number of gene cassettes in the class 1 integrons from koalas and bats ranged from one to four (Table 1).
Twelve different ARGs and two hypothetical proteins (ORFD and ORFX) were identified (Table 1). The majority of integron ARGs conferred resistance to either aminoglycosides (aadA/aadB, n = 6), trimethoprim (dfrA, n = 4), beta-lactams (OXA-2, n = 1) or quaternary ammonium compounds (qacL, n = 1) (Table 1). In koalas, dfrA5/qacEdelta was the most frequent class 1 integron identified (7 of 10) and dfrA17/aadA5/IS26 was the most frequent class 1 integron in bat pups (18 of 36) (Table 1). Four bat pup samples carried two different class 1 integrons containing ARGs, aadA2/qacEdelta plus dfrA17/aadA5/IS26 (n = 3) and aadA2/qacEdelta plus dfrA5/qacEdelta (n = 1) (Supplementary Data 1).
For one integron (aadB/ORFX/OXA-2----aadA1/qacEdelta) the full-length sequence was not obtained due to its long length (~ 3000 bp), with both partial sequences, i.e. the forward sequence (1,164 bp, aadB/ORFX/OXA-2) and the reverse sequence (1,115 bp, aadA1/qacEdelta), returning a 100% match to a class 1 integron (intI1/aadB/ORFX/OXA-2/aadA1/qacEdelta ) from a Corynebacterium amycolatum isolate (GenBank accession AJ871915) (Supplementary Data 1). A BLASTN search of the single ORFX gene resulted in only one match (100% identity), also from the C. amycolatum isolate (GenBank accession AJ871915).
There were 14 samples that were intI1 positive but no ARGs were present in the cassette array. A further 26 samples that were confirmed as intI1 positive by DNA sequencing, either failed to amplify in both the HS458/459 and HS458/JL-D2 PCRs (n = 16), or sequencing identified non-specific PCR products (i.e., non-class 1 integrons) (n = 10). Multiple bands were observed in the HS458/459 and/or HS458/JL-D2 PCRs for 78 intI1 positive samples, in which, cassette array sequencing was not feasible (Supplementary Data 1).
The closest GenBank matches for representative intI1 and class 1 integron sequences from koala and bat DNA samples showed between 99.4% and 100% identity to GenBank accessions (Supplementary Data 1).
Detection of bla TEM Genes in Koala and Bat DNA Samples
The blaTEM gene was detected in 2.6% of all PCR-competent koala DNA samples (7 of 267), with all detections occurring in 2018 at two Mount Lofty Ranges locations (Cleland and Morialta) (Table 1). Of the two koala sampling events where blaTEM was detected, the occurrence ranged from 10.3 to 13.0%, and two blaTEM gene variants were identified, namely blaTEM-116 and blaTEM-1 C (Table 1).
In bat pups, the blaTEM gene was detected in 25.2% of all PCR-competent DNA samples (39 of 155), with detections occurring at all sampled locations, except Bega (Table 1). Of the five bat sampling events where blaTEM was detected, the occurrence ranged from 6.7 to 47.4% (Table 1). Three blaTEM gene variants were identified, with blaTEM-1B being the most frequent (21 of 39) (Table 1). The identity of the blaTEM gene variant could not be determined in 12 samples due to sequence data having multiple peaks (indicative of multiple variants) or insufficient sequence length (Table 1).
The closest GenBank matches for representative blaTEM gene sequences from koala and bat pup DNA samples showed between 99.9% and 100% identity to GenBank accessions (Supplementary Data 1).
Distribution of Class 1 Integrons and bla TEM Genes Across Koala and Bats
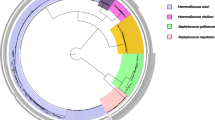
Across all koala and bat samples, 10 distinct class 1 integrons and four distinct blaTEM gene variants were found, of which, eight (four class 1 integrons and all four blaTEM gene variants) were detected in two or more samples, and the remaining six class 1 integrons were detected in only one sample (Table 1; Fig. 1). Of the eight integrons and blaTEM genes detected more than once, four were detected in samples from multiple sampling events (three in both koalas and bats, and one in bats only), and the remaining four were exclusively found in samples from one sampling event (one in koalas and three in bats) (Fig. 1).
The blaTEM-1B gene was most widely distributed and detected in samples from five of six bat sampling events (Fig. 1). The integron arrays aadA2/qacEdelta and dfrA5/qacEdelta were the next most frequent and detected in samples from four sampling events (Fig. 1). The dfrA17/aadA5/IS26 integron was detected in 18 samples, however, distribution was restricted to bats from one sampling event (Adelaide 2018–2019) Fig. 1).
Geographical Clustering of Class 1 Integrons and bla TEM Genes in Mount Lofty Ranges Koalas
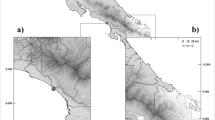
Analysis of Global Positioning System (GPS) location data for all pre-fire (2018) wild-caught Mount Lofty Ranges koalas carrying dfrA5/qacEdelta integrons and blaTEM genes, found all dfrA5/qacEdelta positive koalas (Cleland, n = 7) were clustered within 1.05 km of each other and all blaTEM-116 positive koalas (Cleland, n = 4) were clustered within 1.2 km of each other, with both clusters located in the Cleland Conservation Park (Fig. 2). Notably, one koala (KO4) carried both a dfrA5/qacEdelta integron and a blaTEM-116 gene (Fig. 2). Similarly, all blaTEM-1 C positive koalas (Morialta, n = 3) were clustered within 325 m of each other in the Morialta Conservation Park (Fig. 2).
Satellite map showing the clustered GPS locations of all pre-fire wild-caught Mount Lofty Ranges koalas (Morialta Conservation Park 2018 and Cleland Conservation Park 2018) carrying dfrA5/qacEdelta integrons and blaTEM genes. GPS analysis and satellite map were constructed using Google Earth Pro (Version 7.3.6.9345, Google LLC, Menlo Park, USA).
Association of Wildfires on intI1 and bla TEM Occurrence
For koalas on Kangaroo Island, the occurrence of intI1 was significantly higher in fire-affected koalas that had entered into care (Kangaroo Island 2020) compared to wild pre-fire koalas (Kangaroo Island 2014–2017) (P < 0.0001) (Fig. 3). Similarly, koalas in Belair National Park, the occurrence of intI1 was significantly higher in post-fire koalas (Belair 2022) compared to pre-fire koalas (Belair 2018) (P = 0.0074) (Fig. 3).
The blaTEM gene was only detected in two pre-fire samples (Cleland 2018 and Morialta 2018) (Table 1), of which, neither were re-sampled after the fire 2019/2020 fire season, as such, the association of fire and blaTEM occurrence in koalas could not be tested.
The occurrence of intI1 and blaTEM genes in paired sampling events from koalas in two locations in South Australia, at two time points; Kangaroo Island (KI) pre-fire (2014–2017, n = 46) and fire-affected (2020, n = 32), and Belair National Park pre-fire (2018, n = 29) and post-fire (2022, n = 98). Statistical significance was indicated where P < 0.05 using Fisher’s exact test
To determine the association of fire-season on intI1 and blaTEM occurrence in bat pups, paired samplings collected from the same location at different time points, were evaluated for statistical difference (Fig. 4a and b). In bat pups in Sydney (New South Wales), the occurrence of both intI1 and blaTEM were not significantly different in fire-affected bats (Sydney 2020) compared to pre-fire bats (Sydney 2018–2019) (P > 0.05) (Fig. 4a). In contrast, bats pups in Adelaide (South Australia), the occurrence of intI1 and blaTEM were both significantly higher in pre-fire bats (Adelaide 2018–2019) compared to post-fire bats (Adelaide 2021) (P < 0.05) (Fig. 4b).
Occurrence of intI1 and blaTEM genes in paired sampling events collected from Pteropid bat pups in the same locations at different time points. (a) Sydney pre-fire (2019, n = 19) and fire-affected (2020, n = 11). (b) Adelaide pre-fire (2018–2019, n = 52) and post-fire (2021, n = 30). Statistical significance was indicated where P < 0.05 using Fisher’s exact test
intI1 and bla TEM Occurrence in Wild and In-Care Samples
The association of entry into care on intI1 and blaTEM occurrence in wild koalas and bat pups is significantly higher in both species in-care (Fig. 5). For koalas from Kangaroo Island, the occurrence of intI1 was significantly higher (P < 0.0001) in koalas in-care (Kangaroo Island 2020) compared to wild koalas (Kangaroo Island 2014–2017), while blaTEM was not detected in either wild or in-care samples (Fig. 5a). For fire-affected bat pups, the occurrence of intI1 and blaTEM were both significantly higher (P < 0.05) in bats in-care (Sydney 2020) compared to wild bats (Bega 2019–2020) (Fig. 5b).
Occurrence of intI1 and blaTEM genes in animals in-care compared to animals in the wild a. Kangaroo Island koalas (Wild, 2014–2017, n = 46. In-care, 2020, n = 32). b. Fire-affected Pteropid bat pups (Wild, Bega 2019–2020, n = 37. In-care, Sydney 2020, n = 11). Statistical significance was indicated where P < 0.05 using Fisher’s exact test
Antibiotic Administration and Occurrence of intI1 and bla TEM
Treatment records were available for 27 of 32 Kangaroo Island koalas in-care and for all 118 bat pups in-care. Five Kangaroo Island koalas in-care (Kangaroo Island 2020) and three pre-fire Adelaide bat pups in-care (Adelaide 2018–2019), received antibiotics prior to the collection of samples (Supplementary Data 1). The five koalas received amoxicillin +/- clavulanic acid, with three of five also receiving enrofloxacin, and the three bat pups received amoxicillin + clavulanic acid (Supplementary Data 1). In the Kangaroo Island koalas, the occurrence of intI1 was not significantly different in koalas which received antibiotics (3 of 6, 50.0%) compared to those that did not receive antibiotics (10 of 21, 47.6%) (P = 1.0000). Similarly, for the Adelaide bat pups, there was no significant difference in the occurrence of intI1 in bats which received antibiotics (2 of 3, 66.7%) compared to those that did not receive antibiotics (42 of 49, 85.7%) (p = 0.4007). While blaTEM carriage was higher in the Adelaide bat pups which received antibiotics (3 of 3, 100%) compared to those that did not receive antibiotics (26 of 49, 53.1%), the difference was not statistically significant (P = 0.2455).
Discussion
The frequency of the intI1 gene in koalas and bat pups in this study is consistent with previous reports of intI1 detected in other Australian wildlife species, including the little penguin (E. minor) [26], the brush-tailed rock-wallaby (P. penicillate) [25], Australian marine mammals [4] and adult grey-headed flying foxes (P. poliocephalus) [14]. The diversity of class 1 integrons containing ARGs in koala and bat pups is also consistent with those found in other Australian species, particularly, the high frequency of ARGs conferring resistance to aminoglycosides (aadA) and trimethoprim (dfrA) [4, 14, 25, 26].
This study is the first to report the presence of blaTEM genes in the faecal microbiome of wild koalas. Although the overall occurrence of blaTEM in koala samples was low (2.6%), for two locations the occurrence was 4- to 5-fold higher than the average (10.3% and 13.0%). The detection of three blaTEM gene variants in 25.2% of bat pups is consistent with previous reports of blaTEM genes in Escherichia coli from grey headed flying fox adults and pre-fire pups from Adelaide [6, 7]. The detection of an amoxicillin resistance gene (blaTEM) in koala and bat pup faecal microbiomes is highly concerning given that amoxicillin is a common antibiotic administered to both these species [29].
The bacterial species carrying class 1 integrons and blaTEM genes in koala and bat pup microbiomes were not identified as part of this study and therefore the changing patterns in AMR carriage cannot be linked to bacterial species dynamics. In a previous study where E. coli was cultured from the pre-fire Adelaide bat pup faecal samples used in this study (Adelaide 2018–2019), the dfrA17/aadA5/IS26 class 1 integron was associated with a multidrug resistant E. coli strain ST48 O4:H26 [7] and the blaTEM-33 gene was associated with the beta-lactam resistant E. coli strain ST2144 O166:H49 [7].
The rare class 1 integron, aadB/ORFX/OXA-2—aadA1/qacEdelta, detected in a bat pup under care in a wildlife hospital, matched a single entry in GenBank, a Gram-positive isolate (Corynebacterium amycolatum) [57]. Corynebacterium amycolatum is an opportunistic pathogen associated with urinary tract infections in domestic animals [58, 59] and extraintestinal infections, including blood, endocarditis and peritonitis, in human patients [60, 61]. Notably, class 1 integrons are typically associated with Gram-negative bacteria and rarely with Gram-positive species [62]. Future work to determine if bat pups are carrying an antimicrobial resistant strain of C. amycolatum associated with integron carriage is required.
The clustering of the dfrA17/aadA5/IS26 class 1 integron and the blaTEM-33 gene in specific cohorts of bat pups indicates that they acquired these ARGs from the same environmental source and/or via direct transmission of bacterial strains between co-housed bats as observed previously [7, 16, 63]. In koalas, blaTEM-1 C, blaTEM-116 and dfrA5/qacEdelta appear to also be acquired by wild koalas from the same environmental source given that the GPS locations of wild-caught koalas carrying three ARGs formed three distinct geographical clusters. Direct transmission between koalas is also possible, but less likely compared to potential for transmission between bat pups, given that koalas do not have frequent interactions, except during the breeding season [63, 64].
There was no significant association between antibiotic administration and carriage of intI1 or blaTEM genes in either koalas or bats, however, this may be due to the small sample sizes of koalas and flying foxes which received antibiotics prior to sampling. Concerningly, the three bat pups which received amoxicillin + clavulanic acid were carrying blaTEM genes, of these, two were blaTEM-33 which confers resistance to amoxicillin + clavulanic acid and one pup carried blaTEM-1B which confers resistance to amoxicillin. Amoxicillin and trimethoprim are frequently used to treat infections in koalas [29] and multiple variants of blaTEM and dfrA genes (confer resistance to amoxicillin and trimethoprim respectively) were detected in koalas in this study. The presence of ARGs in wildlife presents a two-fold issue. Firstly, treatment failure may result from unresolved infection with antibiotic resistant bacteria and poor rehabilitation outcome. Secondly, antibiotic administration has the potential to promote the horizontal transfer of ARGs between bacterial species, and risk the emergence of new antimicrobial resistant pathogens [65]. It is imperative that antibiotics are used judiciously, and consideration is given to potential AMR carriage when treating fire-affected koalas and bat pups in-care. Overall, these findings reinforce the need for adherence to antimicrobial stewardship prescribing guidelines [66].
The substantial increase in carriage of intI1 from zero detection in pre-fire koalas to 46.8% in fire-affected koalas in-care, indicates that koalas being driven into care by wildfires is associated with a higher carriage of class 1 integrons. This finding is consistent with previous reports of a higher prevalence of AMR in wildlife in rehabilitation environments, compared to their free-living counterparts [8, 14, 15]. IntI carriage in wild koalas in a fire-impacted environment (Belair National Park), and that did not enter care, was significantly higher in post-fire koalas (32.7%) compared to pre-fire koalas (6.9%), suggesting that wildfires are associated with higher carriage of class 1 integrons at this location.
In contrast to koalas, there was no significant association between wildfires and the carriage of intI1 or blaTEM genes in pre-fire bat pups compared to fire-affected pups. There was a high prevalence of intI1 and blaTEM in pre-fire pups from Sydney in 2018–2019 indicating that increased AMR carriage is also driven by non-fire factors, which may explain why there was no significant increase in intI1 and blaTEM carriage by fire-affected pups. Further, the bats themselves were less likely to be directly impacted by fire unlike koalas. While this study examined fire as a driver of AMR dynamics, the sampling design was opportunistic. Emergency preparedness and study design tailored to wildlife emergencies would support understanding the association between fire and AMR, and the additional risks this poses for wildlife health and conservation. In agreement with koalas, fire-affected bat pups that entered care had a significantly higher carriage of both intI1 and blaTEM genes compared to wild fire-affected pups. These findings further indicate that wildfires are shifting the dynamics of AMR in wildlife when they are driven into care following catastrophic wildfire events.
Multiple intertwined factors can drive acquisition of AMR by fire-affected wildlife that enter care; (1) increased pre-entry exposure to sources of antimicrobial resistant bacteria in fire-impacted environments, for example, via drinking from contaminated water sources [67,68,69], (2) transmission through close connectivity and exposure to wildlife already carrying AMR within wildlife hospital and rehabilitation environments [8, 14, 63], (3) increase in AMR frequency through antibiotic administration to wildlife in-care that may also exacerbate transmission between co-housed wildlife [8, 16, 65] and (4) post-entry exposure to antimicrobial resistant bacteria in human dominated environments [24]. Additionally, previous work has shown that other climate change impacts, including heat stress events in grey headed flying foxes (Pteropid bats), also drives increased prevalence and diversity of AMR in wildlife [7].
Climate change induced extreme weather events including, droughts and heat waves, are predicted to increase over time, and consequently, drive an increased risk of extreme wildfires occurring [36, 70]. Predictions made in the Garnaut Climate Change Review published in 2008 stated that the effect of climate change will be evident in Australia by 2020 with the occurrence of extreme wildfires and earlier and longer fire seasons [71]. Australia will continue to experience extreme weather and wildfires unless the climate crisis is alleviated [71]. These extreme climate change events and wildfires will drive increasing numbers of Australia’s native wildlife into care and rehabilitation [40, 49]. Furthermore, wildfire associated habitat loss and food shortages will continue to impact many threatened species, including koalas and the grey headed flying fox (Pteropid bats), potentially pushing them closer to extinction, unless actions are taken to reduce the risks of future extreme wildfires occurring [32, 38, 72].
While we have investigated the association between the impacts of wildfires on AMR dynamics in Australian wildlife, the escalating risk of wildfires around the globe, and some in critical wildlife habitat [35, 36], presents wider significance for AMR stewardship and ecology in wildlife.
References
Arnold KE, Williams NJ, Bennett M (2016) Disperse abroad in the land’: the role of wildlife in the dissemination of antimicrobial resistance. Biol Lett 12:20160137. https://doi.org/10.1098/rsbl.2016.0137
Vittecoq M, Godreuil S, Prugnolle F, Durand P, Brazier L, Renaud N, Arnal A, Aberkane S, Jean-Pierre H, Gauthier‐Clerc M (2016) Antimicrobial resistance in wildlife. J Appl Ecol 53:519–529. https://doi.org/10.1111/1365-2664.12596
Torres RT, Carvalho J, Cunha MV, Serrano E, Palmeira JD, Fonseca C (2020) Temporal and geographical research trends of antimicrobial resistance in wildlife-a bibliometric analysis. One Health 11:100198. https://doi.org/10.1016/j.onehlt.2020.100198
Fulham M, McDougall F, Power M, McIntosh RR, Gray R (2022) Carriage of antibiotic resistant bacteria in endangered and declining Australian pinniped pups. PLoS ONE 17:e0258978. https://doi.org/10.1371/journal.pone.0258978
Mukerji S, Stegger M, Truswell AV, Laird T, Jordan D, Abraham RJ, Harb A, Barton M, O’Dea M, Abraham S (2019) Resistance to critically important antimicrobials in Australian silver gulls (Chroicocephalus novaehollandiae) and evidence of anthropogenic origins. J Antimicrob Chemother 74:2566–2574. https://doi.org/10.1093/jac/dkz242
McDougall FK, Boardman WS, Power ML (2021) Characterization of beta-lactam-resistant Escherichia coli from Australian fruit bats indicates anthropogenic origins. Microb Genomics 7:000571. https://doi.org/10.1099/mgen.0.000571
McDougall F, Boardman W, Power M (2022) High prevalence of beta-lactam-resistant Escherichia coli in South Australian grey-headed flying fox pups (Pteropus poliocephalus). Microorg 10:1589. https://doi.org/10.3390/microorganisms10081589
Blyton MD, Pi H, Vangchhia B, Abraham S, Trott DJ, Johnson JR, Gordon DM (2015) Genetic structure and antimicrobial resistance of Escherichia coli and cryptic clades in birds with diverse human associations. Appl Environ Microbiol 81:5123–5133. https://doi.org/10.1128/AEM.00861-15
Smith HG, Bean DC, Clarke RH, Loyn R, Larkins JA, Hassell C, Greenhill AR (2022) Presence and antimicrobial resistance profiles of Escherichia coli, Enterococcus spp. and Salmonella sp. in 12 species of Australian shorebirds and terns. Zoonoses Publ Health 69:615–624. https://doi.org/10.1111/zph.12950
Dolejska M, Masarikova M, Dobiasova H, Jamborova I, Karpiskova R, Havlicek M, Carlile N, Priddel D, Cizek A, Literak I (2015) High prevalence of Salmonella and IMP-4-producing Enterobacteriaceae in the silver gull on Five Islands, Australia. J Antimicrob Chemother 71:63–70. https://doi.org/10.1093/jac/dkv306
Tardón A, Bataller E, Llobat L, Jiménez-Trigos E (2021) Bacteria and antibiotic resistance detection in fractures of wild birds from wildlife rehabilitation centres in Spain. Comp Immunol. Microbiol Infect Dis 74:101575. https://doi.org/10.1016/j.cimid.2020.101575
Zhou W, Zhu L, Jia M, Wang T, Liang B, Ji X, Sun Y, Liu J, Guo X (2018) Detection of multi-drug-resistant Escherichia coli in a giant panda (Ailuropoda melanoleuca) with extraintestinal polyinfection. J Wildl Dis 54:626–630. https://doi.org/10.7589/2017-08-196
Lee JI, Kim KS, Oh BC, Kim NA, Kim IH, Park CG, Kim SJ (2011) Acute necrotic stomatitis (noma) associated with methicillin-resistant Staphylococcus aureus infection in a newly acquired rhesus macaque (Macaca mulatta). J Med Primatol 40:188–193. https://doi.org/10.1111/j.1600-0684.2011.00470.x
McDougall F, Boardman W, Gillings M, Power M (2019) Bats as reservoirs of antibiotic resistance determinants: a survey of class 1 integrons in Grey-headed Flying foxes (Pteropus poliocephalus). Infect Genet Evol 70:107–113. https://doi.org/10.1016/j.meegid.2019.02.022
Kinjo T, Minamoto N, Sugiyama M, Sugiyama Y (1992) Comparison of antimicrobial resistant Escherichia coli in wild and captive Japanese serows. J Vet Med Sci 54:821–827. https://doi.org/10.1292/jvms.54.821
Ishihara K, Hosokawa Y, Makita K, Noda J, Ueno H, Muramatsu Y, Mukai T, Yamamoto H, Ito M, Tamura Y (2012) Factors associated with antimicrobial-resistant Escherichia coli in zoo animals. Res Vet Sci 93:574–580. https://doi.org/10.1016/j.rvsc.2011.09.006
Ahmed AM, Motoi Y, Sato M, Maruyama A, Watanabe H, Fukumoto Y, Shimamoto T (2007) Zoo animals as reservoirs of gram-negative bacteria harboring integrons and antimicrobial resistance genes. Appl Environ Microbiol 73:6686–6690. https://doi.org/10.1128/AEM.01054-07
Partridge SR, Kwong SM, Firth N, Jensen SO (2018) Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev 31:e00088–e00017. https://doi.org/10.1128/cmr.00088-17
Gillings M, Boucher Y, Labbate M, Holmes A, Krishnan S, Holley M, Stokes HW (2008) The evolution of class 1 integrons and the rise of antibiotic resistance. J Bacteriol 190:5095–5100. https://doi.org/10.1128/jb.00152-08
Stokes HW, Gillings MR (2011) Gene flow, mobile genetic elements and the recruitment of antibiotic resistance genes into Gram-negative pathogens. FEMS Microbiol Rev 35:790–819. https://doi.org/10.1111/j.1574-6976.2011.00273.x
Hall RM, Collis CM (1995) Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol Microbiol 15:593–600. https://doi.org/10.1111/j.1365-2958.1995.tb02368.x
Dawes FE, Kuzevski A, Bettelheim KA, Hornitzky MA, Djordjevic SP, Walker MJ (2010) Distribution of class 1 integrons with IS26mediated deletions in their 3′-conserved segments in Escherichia coli of human and animal origin. PLoS ONE 5:e12754. https://doi.org/10.1101/158808
Gillings MR (2018) DNA as a pollutant: the clinical class 1 Integron. Curr Pollut Rep 1–7. https://doi.org/10.1007/s40726-018-0076-x
Skurnik D, Ruimy R, Andremont A, Amorin C, Rouquet P, Picard B, Denamur E (2006) Effect of human vicinity on antimicrobial resistance and integrons in animal faecal Escherichia coli. J Antimicrob Chemother 57:1215–1219. https://doi.org/10.1093/jac/dkl122
Power ML, Emery S, Gillings MR (2013) Into the wild: dissemination of antibiotic resistance determinants via a species recovery program. PLoS ONE 8:e63017. https://doi.org/10.1371/journal.pone.0063017
Lundbäck IC, McDougall FK, Dann P, Slip DJ, Gray R, Power ML (2021) Into the sea: antimicrobial resistance determinants in the microbiota of little penguins (Eudyptula minor). Infect Genet Evol 88:104697. https://doi.org/10.1016/j.meegid.2020.104697
Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, Laxminarayan R (2014) Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 14:742–750. https://doi.org/10.1016/S1473-3099(14)70780-7
De Briyne N, Atkinson J, Borriello S, Pokludová L (2014) Antibiotics used most commonly to treat animals in Europe. Vet Rec 175:325–325. https://doi.org/10.1136/vr.102462
Bodley K (2019) Appendix 4. Drug formulary. In: Vogelnest L, Portas T (eds) Current therapy in Medicine of Australian mammals. CSIRO Publishing, Clayton South (VIC), pp 702–727
Shahid M, Singh A, Sami H (2022) Beta-Lactam Resistance in Gram-negative Bacteria: threats and challenges. Springer Nature, Singapore
Narciso-da-Rocha C, Varela AR, Schwartz T, Nunes OC, Manaia CM (2014) blaTEM and vanA as indicator genes of antibiotic resistance contamination in a hospital–urban wastewater treatment plant system. J Glob Antimicrob Resist 2:309–315. https://doi.org/10.1016/j.jgar.2014.10.001
Wintle BA, Legge S, Woinarski JC (2020) After the megafires: what next for Australian wildlife? Trends Ecol Evol 35:753–757. https://doi.org/10.1016/j.tree.2020.06.009
Khan SJ (2021) Ecological consequences of Australian black summer(2019–20) fires: a synthesis of Australian Commonwealth Government report findings. Integr Environ Assess Manag 17:1136–1140. https://doi.org/10.1002/ieam.4469
World Wildlife Fund Australia (2020) Australia’s 2019–2020 Bushfires: The Wildlife Toll. World Wide Fund for Nature Australia. https://www.wwf.org.au/what-we-do/bushfire-recovery/in-depth/resources/australia-s-2019-2020-bushfires-the-wildlife-toll#gs.9uc8ky. Accessed 12 June 2023
OECD (2023) Taming wildfires in the Context of Climate Change. OECD Publishing, Paris. https://doi.org/10.1787/dd00c367-en
Jones MW, Abatzoglou JT, Veraverbeke S, Andela N, Lasslop G, Forkel M, Smith AJ, Burton C, Betts RA, van der Werf GR (2022) Global and regional trends and drivers of fire under climate change. Rev Geophys 60. https://doi.org/10.1029/2020RG000726. e2020RG000726
Dunstan E, Funnell O, McLelland J, Stoeckeler F, Nishimoto E, Mitchell D, Mitchell S, McLelland DJ, Kalvas J, Johnson L (2021) An analysis of demographic and triage assessment findings in bushfire-affected koalas (Phascolarctos cinereus) on Kangaroo Island, South Australia, 2019–2020. Animals 11:3237. https://doi.org/10.3390/ani11113237
Phillips S, Wallis K, Lane A (2021) Quantifying the impacts of bushfire on populations of wild koalas (Phascolarctos cinereus): insights from the 2019/20 fire season. Ecol Manage Restor 22:80–88. https://doi.org/10.1111/emr.12458
Parrott ML, Wicker LV, Lamont A, Banks C, Lang M, Lynch M, McMeekin B, Miller KA, Ryan F, Selwood KE (2021) Emergency response to Australia’s black summer 2019–2020: the role of a zoo-based conservation organisation in wildlife triage, rescue, and resilience for the future. Animals 11:1515. https://doi.org/10.3390/ani11061515
Lunney D, Cope H, Sonawane I, Haering R (2022) A state-wide picture of koala rescue and rehabilitation in New South Wales during the 2019–2020 bushfires. Aust Zool 42:243–255. https://doi.org/10.7882/AZ.2022.013
Government of South Australia (2020) Independent Review into South Australia’s 2019-20 Bushfire Season. https://safecom-files-v8.s3.amazonaws.com/current/docs/Independent%2520Review%2520into%2520SA%2527s%25202019-20%2520Bushfire%2520Season%2520-%2520Web%2520Upload.pdf. Accessed 12 June 2023
Australian Government (1999) Environment Protection and Biodiversity Conservation Act. Commonwealth of Australia, Canberra (ACT). https://www.environment.gov.au/cgi-bin/sprat/public/publicspecies.pl?taxon_id=85104. Accessed 12 June 2023
Australian Government (2021) National recovery plan for the grey-headed flying fox Pteropus poliocephalushttps://www.awe.gov.au/environment/biodiversity/threatened/publications/recovery/grey-headed-flying-fox (Accessed 12 June 2023)
Welbergen JA, Meade J, Field HE, Edson D, McMichael L, Shoo LP, Praszczalek J, Smith C, Martin JM (2020) Extreme mobility of the world’s largest flying mammals creates key challenges for management and conservation. BMC Biol 18:1–13. https://doi.org/10.1186/s12915-020-00829-w
Nelson JE (1965) Behaviour of Australian pteropodidae (Megacheroptera). Anim Behav 13:544–557. https://doi.org/10.1016/0003-3472(65)90118-1
Eby P (1991) Seasonal movements of grey-headed flying-foxes, Pteropus poliocephalus (Chiroptera: Pteropodidae), from two maternity camps in northern New South Wales. Wildl Res 18:547–559. https://doi.org/10.1071/WR9910547
Eby P, Roberts B, Pennay M, Welbergen JA (2021) Pteropus poliocephalus. The IUCN Red List of Threatened Species 2021: e.T18751A22085511. https://doi.org/10.2305/IUCN.UK.2021-3.RLTS.T18751A22085511.en. Accessed on 12 June 2023
Mo M, Minehan M, Hack E, Place V, Welbergen JA (2022) A report of direct mortality in grey-headed flying-foxes (Pteropus poliocephalus) from the 2019–2020 Australian megafires. Australian Mammalogy. https://doi.org/10.1071/AM21041
Mo M, Roache M, Davies J, Hopper J, Pitty H, Foster N, Guy S, Parry-Jones K, Francis G, Koosmen A (2021) Estimating flying-fox mortality associated with abandonments of pups and extreme heat events during the austral summer of 2019–20. Pac Conserv Biol 28:124–139. https://doi.org/10.1071/PC21003
Fabijan J, Caraguel C, Jelocnik M, Polkinghorne A, Boardman WS, Nishimoto E, Johnsson G, Molsher R, Woolford L, Timms P (2019) Chlamydia pecorum prevalence in South Australian koala (Phascolarctos cinereus) populations: identification and modelling of a population free from infection. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-42702-z
Lane D (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. John Wiley and Sons, New York, NY, pp 115–147
McDougall F, Gordon D, Robins-Browne R, Bennett-Wood V, Boardman WS, Graham PL, Power M (2023) Characterisation of typical enteropathogenic Escherichia coli (tEPEC) lineages and novel bfpA variants detected in Australian fruit bats (Pteropus poliocephalus). Sci Total Environ 902:166336. https://doi.org/10.1016/j.scitotenv.2023.166336
Waldron LS, Gillings MR (2015) Screening foodstuffs for class 1 Integrons and Gene Cassettes. J Visualized Experiments: e 52889–e52889. https://doi.org/10.3791/52889
Holmes AJ, Gillings MR, Nield BS, Mabbutt BC, Nevalainen K, Stokes H (2003) The gene cassette metagenome is a basic resource for bacterial genome evolution. Environ Microbiol 5:383–394. https://doi.org/10.1046/j.1462-2920.2003.00429.x
Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwarz S, Cattoir V, Philippon A, Allesoe RL, Rebelo AR, Florensa AF (2020) ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother 75:3491–3500. https://doi.org/10.1093/jac/dkaa345
Pitout J, Thomson K, Hanson N, Ehrhardt A, Moland E, Sanders C (1998) β-Lactamases responsible for resistance to expanded-spectrum cephalosporins in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis isolates recovered in South Africa. Antimicrob Agents Chemother 42:1350–1354. https://doi.org/10.1128/aac.42.6.1350
Ploy M-C, Gassama A, Chainier D, Denis F (2005) Les intégrons en tant que support génétique de résistance aux antibiotiques. Immuno-analyse & Biologie spécialisée 20:343–352. https://doi.org/10.1016/j.immbio.2005.10.001
Poor AP, Moreno LZ, Matajira CE, Parra BM, Gomes VT, Silva APS, Dutra MC, Christ APG, Barbosa MR, Sato MIZ (2017) Characterization of Corynebacterium diphtheriae, C. Confusum and C. Amycolatum isolated from sows with genitourinary infection. Vet Microbiol 207:149–152. https://doi.org/10.1016/j.vetmic.2017.06.008
Rogovskyy AS, Bailey LK, Blazier JC, Cai JJ, Landis M, Lidbury JA, Pavlova E, Wu J (2022) The brief case: Corynebacterium amycolatum in a relapsing urinary tract infection of a Feline patient. J Clin Microbiol 60:e00453–e00421. https://doi.org/10.1128/JCM.00453-21
Knox KL, Holmes AH (2002) Nosocomial endocarditis caused by Corynebacterium amycolatum and other nondiphtheriae corynebacteria. Emerg Infect Dis 8:97. https://doi.org/10.3201/eid0801.010151
Bernard K (2012) The genus Corynebacterium and other medically relevant coryneform-like bacteria. J Clin Microbiol 50:3152–3158. https://doi.org/10.1128/jcm.00796-12
Partridge SR, Tsafnat G, Coiera E, Iredell JR (2009) Gene cassettes and cassette arrays in mobile resistance integrons. FEMS Microbiol Rev 33:757–784. https://doi.org/10.1111/j.1574-6976.2009.00175.x
Mühldorfer K (2013) Bats and bacterial pathogens: a review. Zoonoses Publ Health 60:93–103. https://doi.org/10.1111/j.1863-2378.2012.01536.x
Watchorn DJ, Whisson DA (2019) Quantifying the interactions between koalas in a high-density population during the breeding period. Australian Mammalogy 42:28–37. https://doi.org/10.1071/AM18027
Raplee I, Walker L, Xu L, Surathu A, Chockalingam A, Stewart S, Han X, Rouse R, Li Z (2021) Emergence of nosocomial associated opportunistic pathogens in the gut microbiome after antibiotic treatment. Antimicrob Resist Infect Control 10:1–11. https://doi.org/10.1186/s13756-021-00903-0
Power M (2019) Chap. 17. Antimicrobial resistance. In: Vogelnest L, Portas T (eds) Current therapy in Medicine of Australian mammals. CSIRO Publishing, Clayton South (VIC), pp 285–292
Magnano San Lio R, Favara G, Maugeri A, Barchitta M, Agodi A (2023) How antimicrobial resistance is linked to climate change: an overview of two intertwined global challenges. Int J Environ Res Public Health 20:1681. https://doi.org/10.3390/ijerph20031681
Mishra A, Alnahit A, Campbell B (2020) Impact of land uses, drought, flood, wildfire, and cascading events on water quality and microbial communities: a review and analysis. J Hydrol 125707. https://doi.org/10.1016/j.jhydrol.2020.125707
Caruso B (2002) Temporal and spatial patterns of extreme low flows and effects on stream ecosystems in Otago, New Zealand. J Hydrol 257:115–133. https://doi.org/10.1016/S0022-1694(01)00546-7
Duane A, Castellnou M, Brotons L (2021) Towards a comprehensive look at global drivers of novel extreme wildfire events. Clim Change 165:43. https://doi.org/10.1007/s10584-021-03066-4
Garnaut R (2008) The Garnaut climate change review. Cambridge, Cambridge
Baranowski K, Faust CL, Eby P, Bharti N (2021) Quantifying the impacts of Australian bushfires on native forests and gray-headed flying foxes. Glob Ecol Conserv 27:e01566. https://doi.org/10.1016/j.gecco.2021.e01566
Acknowledgements
We wish to thank the flying fox carers from Fauna Rescue of South Australia, NSW Wildlife Information Rescue and Education Service (WIRES), The Shoalhaven Bat Clinic (Wildlife Rescue South Coast), Taronga Wildlife Hospital Sydney, Hugh Pitty and other flying fox carers for collecting faecal samples from grey-headed flying fox pups. We wish to thank Claire Moore, Karen Burke de Silva and Julian Beaman (Flinders University) and the DVM students (School of Animal and Veterinary Sciences, University of Adelaide) for the collection of koala faecal samples in Belair National Park in October 2022. We wish to thank Jen McLelland and Ian Smith (Zoos South Australia), the Kangaroo Island Veterinary Clinic, and all emergency veterinarians, personnel and volunteers on Kangaroo Island during the 2019-2020 wildfires for collecting samples from fire-affected Kangaroo Island koalas. We also wish to thank Tamsyn Stephenson (University of Adelaide) for the collection and processing of Kangaroo Island koala samples. This study was made possible with support from Morris Animal Foundation, whose mission is to bridge science and resources to advance the health of animals. Morris Animal Foundation is a 501(c)3 nonprofit organization. The Foundation relies upon donations from animal lovers and veterinarians, as well as nonprofit and for-profit organizations, to accelerate urgently needed breakthroughs in veterinary medicine. Visit morrisanimalfoundation.org to learn more. We acknowledge the Wallumattagal clan of the Dharug Nation as the original custodians of the Macquarie University land where laboratory analyses were performed, the Kaurna People as original custodians of the lands where the University of Adelaide campuses are built, the Ngarrindjeri and Kaurna Peoples as original custodians of the lands where samples were collected in South Australia, and all original custodians of the lands where samples were collected in New South Wales.
Funding
The study was funded by the Morris Animal Foundation (Grant No. D21ZO-507 to Michelle Power and Grant No. D16ZO-829 and D21ZO-506 to Natasha Speight).
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
FM, MP and WB contributed to the study conception and design. FM and MP developed the study methodologies. Sample acquisition and data collection was performed by all authors. DNA extraction of samples was performed by FM and NS. Molecular analysis of samples was performed by FM. Data curation was performed by FM, WB, OF and NS. Data analysis was performed by FM. The first draft of the manuscript was written by FM and all authors contributed to the manuscript review and editing. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Animal Ethics
Mounty Lofty Ranges koala sample collection in 2018 was approved by The University of Adelaide Animal Ethics Committee approval number S-2018-022 and the South Australian Department for Environment and Water (DEW) Scientific Research permit number Y26054-7. Kangaroo Island pre-fire koala sample collection was approved by The University of Adelaide Animal Ethics Committee approval number S-2013-198 and the South Australian Department for Environment and Water (DEW) Scientific Research permit number Y26054-6. Belair National Park koala sample collection in 2022 was approved by Flinders University Animal Ethics Committee approval number AEC BIOL 4222-13 and the South Australian Department for Environment and Water (DEW) Scientific Research permit number U27069. The acquisition of archived chlamydial disease diagnostic samples collected from koalas in veterinary care on Kangaroo Island and in mainland Australia was approved under the Macquarie University Animal Ethics Committee (ARA permit Reference No. 2020/027 − 2) permitting the collection samples from koalas in-care. Flying fox sample collection was conducted with approval from the animal ethics committees at Macquarie University (No. 2017/013) and the University of Adelaide (No. S-2015-028), along with a South Australia Department of Environment and Water Wildlife Scientific Permit (M-23671-1, 2 and 3) and an Opportunistic Sample Licence Agreement (R18B259) with Taronga Conservation Society Australia.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McDougall, F.K., Speight, N., Funnell, O. et al. Dynamics of Antimicrobial Resistance Carriage in Koalas (Phascolarctos Cinereus) and Pteropid Bats (Pteropus Poliocephalus) Before, During and After Wildfires. Microb Ecol 87, 39 (2024). https://doi.org/10.1007/s00248-024-02351-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00248-024-02351-w