Abstract
Roe deer (Capreolus capreolus) are found in various habitats, from pure forest cultures to agricultural areas and mountains. In adapting to the geographically and seasonally differentiating food supply, they depend, above all, on an adapted microbiome. However, knowledge about the microbiome of wild ruminants still needs to be improved. There are only a few publications for individual species with a low number of samples. This study aims to identify a core microbiota for Bavarian roe deer and present nutrient and microbiota portraits of the individual habitat types. This study investigated the roe deer’s rumen (reticulorumen) content from seven different characteristic Bavarian habitat types. The focus was on the composition of nutrients, fermentation products, and the rumen bacterial community. A total of 311 roe deer samples were analysed, with the most even possible distribution per habitat, season, age class, and gender. Significant differences in nutrient concentrations and microbial composition were identified for the factors habitat, season, and age class. The highest crude protein content (plant protein and microbial) in the rumen was determined in the purely agricultural habitat (AG), the highest value of non-fibre carbohydrates in the alpine mountain forest, and the highest fibre content (neutral detergent fibre, NDF) in the pine forest habitat. Maximum values for fibre content go up to 70% NDF. The proportion of metabolites (ammonia, lactate, total volatile fatty acids) was highest in the Agriculture-Beech-Forest habitat (ABF). Correlations can be identified between adaptations in the microbiota and specific nutrient concentrations, as well as in strong fluctuations in ingested forage. In addition, a core bacterial community comprising five genera could be identified across all habitats, up to 44% of total relative abundance. As with all wild ruminants, many microbial genera remain largely unclassified at various taxonomic levels. This study provides a more in-depth insight into the diversity and complexity of the roe deer rumen microbiota. It highlights the key microorganisms responsible for converting naturally available nutrients of different botanical origins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The roe deer (Capreolus capreolus) has evolved in recent decades from a typical forest edge and scrub habitat dweller to an inhabitant of diverse habitat types [1, 2]. As a typical synanthropic species, it increasingly uses agricultural habitats, where the energy supply is sometimes even higher than in pure forest areas and to which it is optimally adapted to browse [3, 4]. Nevertheless, various types of forest, alpine areas, or grassland farming are also used by roe deer. The diverse habitats give the animals different forage offers, both seasonally and regionally, because of the plant availability and nutrient composition [3, 5, 6].
The essential tool for adaptation to these conditions is the ruminal microbiome of the roe deer, which is the key for the ruminant to access energy and nutrients from the plants. The rumen forms the first anaerobic digestive chamber and is inhabited by a diverse microbial community. The bacteria and fungi living in it provide the required carbohydrate-active enzymes for the hydrolysation of complex polysaccharides. The following microbial fermentation processes generate volatile fatty acids (VFAs), which the host uses as a primary energy source [7, 8]. In addition to active fermentation, the microbiome also serves as an essential non-vegetable protein source for the host forming the central part of the protein that the host can digest. Most of the protein supplied through food intake is converted directly into ammonia and is then used to build microbial protein [9].
The roe deer is a selector [4] and can efficiently break down and utilise high fibre content in the ingested forage. Thanks to microbial plasticity, they are largely resistant to changes in diet composition [3]. It shows snacking habits and consumes many different plants per browsing period. It prefers young shoots, buds, shrub fruits, tree fruits, and herbs, if available [10]. However, grass and woody plant parts are also integral to the diet. Over 300 plant species are known to be ingested by the deer [6, 11, 12], which adapts to the seasonal and local supply. The roe deer takes its forage, distributed over the day, in an average of 8–11 browsing periods [11, 12]. The passage rate is, therefore, much faster than for typical grazers [11]. In times of lower or less diverse food availability, however, it can also adapt to even more fibre-rich food, which has to stay longer in the rumen. One adaptation mechanism is, for example, the increased filling of the rumen per browsing period in winter and the resulting longer digestion and rumination times [4].
The degree of tolerance to the fibre content in selectors (or browsers) is still highly discussed. Although there is sufficient evidence to the contrary, it is still often said that selectors can only utilise small amounts of fibre and depend on forage with a very high protein and energy content, even in scientific circles. Browsing areas are often evaluated only based on the protein content of the plants. It was also assumed for a long time that selectors such as roe deer had hardly any cellulose-utilising bacteria in their rumen [13]. But fortunately, the point of view has slowly changed. The rigid picture of categorising ruminants as roughage eaters, intermediate types, and concentrate selectors [11] is being replaced by the knowledge that the transitions between the individual feeding types are fluid. Therefore, we can speak of a “browser-grazer continuum” [14]. However, the field of wildlife nutrition continues to offer much potential for further research.
The extent to which ruminants adapt smoothly to nutritional changes is known mainly from numerous dairy cow, beef cattle, and sheep studies [15,16,17]. Literature on wild ruminants is rather limited and often based on minimum sample size [18]. In addition, the correct identification and functional assignment of individual bacterial genera of the microbiota is not trivial, as many species still need to be isolated and described [19]. So, there is still an enormous gap in knowledge in this field.
This study aims to identify the core microbiota of Bavarian roe deer and promote a basis for future comparability of wildlife studies. The second aim was to describe the variations of the rumen microbiota and its fermentation products of free-living roe deer from seven different characteristic Bavarian habitat types throughout the year. When communicating with parties involved in wildlife management (such as foresters, hunters, or farmers), it is often argued that results from other habitats are not comparable to their local situation. To avoid this, the most characteristic and extreme Bavarian habitat communities from different growth areas were selected in this study to present a comprehensive picture.
We hypothesise that a dynamic adaptation of the nutritional conditions is reflected by changes in the rumen microbiota depending on the respective ecological habitat.
Materials and Methods
Sample Material and Study Areas
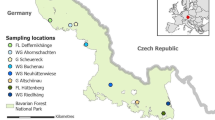
Within the framework of this study, roe deer samples were collected from seven different project areas (Table 1). The seven areas represent the most typical habitat types in Bavaria (Germany). They cover habitats with extreme conditions (in terms of climate or forage availability), such as pine forests, mountains, or pure agricultural landscapes, as well as typical forest communities and mixed forms with agricultural and forest components (like habitat ABF). They also show their primary occurrence at the selected sample sites, and each harbours large roe deer populations. Besides differences in altitude, geological aspects, and local climatic parameters (Table S3), habitats differ primarily in the composition of the plant community and, thus, the available forage for wildlife. For more habitat information, see Table 1 and Table S3 (suppl. information). Five habitats are managed by the Bayerische Staatsforsten AöR (Bavarian Forestry Authority, a public-law institution), one by the University of Würzburg, and one privately. The areas within the habitats were selected with regard to accessibility, on-site support, and the absence of anthropogenic feeding. Offered feed is only found in the form of apple pomace for attraction during hunting (autumn/winter).
All roe deer samples were obtained during regular hunting activities and shooting schedules. To ensure continuous sampling throughout the year, closed seasons, variable by forestry, were suspended for males and subadult females between November and May. After the culling, the entire rumen (reticulorumen) with its contents and other organs were removed and packed in plastic bags. The samples were frozen to at least −18C° as soon as possible after collection. In contrast to experiments with domestic ruminants under controlled conditions, sampling is impossible within a few minutes in the context of hunting activity. On average, 30–60 min can elapse between shooting, organ removal, and freezing. The samples were almost exclusively obtained during individual hunts, which allows for faster sampling than during social hunts. This is an unavoidable bias in wildlife sampling.
Furthermore, the condition and constitution data of each animal were recorded. The age was determined based on tooth development (in young animals) or the tooth section method [20] in older animals (from the age of 2) and was divided into age classes (juvenile, subadult, adult) (distribution of frequencies in Table S2). For the microbiome studies, 311 roe deer from all ages and both gender were sampled. Samples for the spruce forest (SF) and agriculture (AG) habitats were collected in 2011–2014 as part of the preliminary study [6], and all other samples between 2017 and 2019 as part of the ongoing. Information regarding vegetation surveys and botanical rumen content analyses are given in Supplementary Information (Fig. S1) and were previously published [5]. Additional information on the climate data of the individual habitats is shown in Table S3.
The samples were thawed for further processing in the laboratory. The rumen was separated and opened, and the complete content (solid and liquid content) was homogenised by stirring. Approximately 500ml of homogenised content was removed and refrozen. For the separation of 1.5ml for DNA extraction, the content was later thawed and homogenised again. After the separation of material for DNA extraction, 10–15ml of supernatant rumen juice was filled or, if necessary, passed through filter paper to analyse metabolites and refrozen.
Analyses of Crude Nutrients and Fermentation Products
The thawed rumen contents (ca. 500ml) were centrifuged (4400 × g, 15 min, 21°C), and the precipitate was freeze-dried and ground to 1mm grain size.
The crude nutrients [%/DM] (crude protein (CP), total lipids (TL), ash, acid detergent fibre (ADF (ADFom, after ashing)), neutral detergent fibre (NDF, also referred to as “total fibres” in the text (aNDFom, after ashing and amylase treatment)), and acid detergent lignin (ADL/lignin) were analysed, and the fibre fractions were calculated using standard feed analysis procedures. Hemicellulose results from the difference between NDF and ADF, and cellulose from the difference between ADF and lignin. In addition, the crude fibre content (CF) was analysed for comparison with older studies. The proportion of non-fibre-carbohydrates (NFC) was calculated from the difference between the dry masses and the other crude nutrients (Weender and VanSoest analysis; Methods 3.1, 4.1.1, 5.1.1, 6.1.1, 8.1, 6.5.1, 6.5.2, 6.5.3; VDLUFA 2012) [21].
The rumen liquid was centrifuged, and 10 ml was used to analyse the fermentation products. The ammonia and lactate content was determined by photometric measurement at 340 nm. For ammonia, the test kit of Randox (Randox Laboratories Ltd., Crumlin, United Kingdom, Manual AM 1015) was used. The test kit of Boehringer Mannheim/R-Biopharm AG (Darmstadt, Germany) was used for lactate.
The volatile fatty acids (VFAs: acetic (AA), propionic (PA), butyric (BA), valeric (VA), isobutyric (IBA) and isovaleric acid (IVA)) were analysed using a gas chromatograph (Perkin Elmer, Clarus 580, Waltham, Massachusetts). As an internal standard, 100 μl of 2-methyl valeric acid was diluted with 250 ml of 2% metaphosphoric acid for calibration.
DNA Extraction and Illumina Amplicon Sequencing
According to Burbach et al. [22], DNA extraction was performed using the FastDNATM SPIN Kit for Soil (MP Biomedical, Solon, OH, USA) and 200–250 mg of homogenised rumen content as starting material. The quality and purity of the DNA extracts were checked using the NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Amplicon library preparation was performed according to Kaewtapee et al. [23] and targeting the V1-2 region of the 16S rRNA gene. Each PCR reaction mixture (20 μl) consisted of 4 μl 5x Prime Star buffer (TaKaRa Bio Inc., Kusatsu, Japan), 1.6 μl deoxynucleoside triphosphate mixture, each 0.5 μl primer (1:10 diluted), 0.2 μl PrimeSTAR HS DNA Polymerase (250U, TaKaRa Bio Inc., Kusatsu, Japan), 1 μl enhancer (BioStab PCR optimiser (II) 53833-5ML-F, Sigma-Aldrich®, Merck KGaA, Darmstadt, Germany) (only used for PCR 1), and 1 μl template DNA.
The first PCR started with an initial temperature of 95°C for 3 min, followed by 15 cycles of denaturation at 98°C for 10 s, annealing at 55°C for 10 s, extension at 72°C for 45 s, and a final extension at 72°C for 2 min. Next, 1μl of the first PCR was used for the reaction mixture of the second PCR with 20 cycles, following the same PCR conditions. The reaction mixture also contained 10 μl 5× PrimeStar Buffer, 4 μl dNTP mixture, each 1.25 μl primer (1:10), and 0.5 μl polymerase (50μl in total). Due to the nature of the samples, some samples required a pre-PCR step (10 cycles) to ensure the correct amplification.
The expected amplicons were confirmed using gel electrophoresis, normalised using SequalPrep™ Normalization Kit (Applied Biosystems) and purified using MinElute PCR Purification Kit (Qiagen), and sequenced using 250 bp paired-end sequencing chemistry on Illumina NovaSeq 6000.
Raw sequences were demultiplexed with Sabre1 and processed by Qiime2 (v.2023.5) [24]. The q2-cutadapt plugin was used to remove primers [22]. Reads were quality filtered, error corrected, dereplicated, and merged by the q2-dada2 plugin [25]. Taxonomy assignment of generated amplicon sequence variants (ASVs) was implemented in VSEARCH-based consensus and pre-fitted sklearn-based classifiers against the Silva SSU-rRNA database (v.138.1, 16S 99%) [26]. Unassigned sequences and the reads from organelles were removed. The q2-phylogeny plugin was utilised to construct a phylogenetic tree, employing MAFFT 7.3 [27] and FastTree 2.1 [28]. The phylogenetic tree, feature table, and taxonomy table were output for further statistics analysis. After filtering the data, 17,539 ASVs and 309 samples remain for further calculation.
Statistics and Data Analysis
R version 4.3.0 was used for statistical analysis and visualisation [29]. For diversity assessment, ASV tables were rarefied to 10,000 sampling depths. Alpha diversity was estimated by Shannon’s entropy indices, and Bray–Curtis distances were calculated for beta diversity using the phyloseq R package [30]. A principal-coordinate analysis (PCoA) was utilised to ordinate the beta-diversity distances. Alpha diversity results were tested with the Wilcoxon rank-sum test, while the PERMANOVA test was used for beta diversity with 999 permutations by using Vegan R package [31]. The adjusted p-values were corrected for multiple comparisons using the Benjamini–Hochberg procedure. Taxonomy plot and statistics results were visualised by ggplot2 [32]. UpSet R package was used to find taxa similarity across all samples [33]. The core microbiota was identified if a genus was detected with a relative abundance of at least 1 % and 70% occurrence across all samples.
The MaAsLin2 was used to determine the association between core taxa relative abundance and crude nutrients and fermentation products. The measured values of crude protein, NFC, NDF, ammonia, AA, PA, BA, VA, IBA, and IVA were included as fixed effects [34]. SPSS (IBM SPSS Statistics Version 27.0.1.0) was used to compare the abundance means of the crude nutrients, fermentation products and microbial genera between the habitats and seasons statistically. The normal distributions were determined by the Shapiro-Wilk test. The Kruskal-Wallis analysis with post hoc Bonferroni was used for pairwise comparison of the abundance means.
Results
Crude Nutrients
All nutrient groups show significant differences in relation to the habitat factor (p < 0.001, Total lipids p = 0.037). However, the significant difference is only caused by a few habitats per nutrient group. An example of this is a significantly high crude protein content in the AG and SF habitats, a significantly high NFC content in the alpine habitats, or a significantly high total fibre content in the PF, BF, and SF forest habitats (Fig. 1, Table S4). The situation is similar for the seasonal factor (p ≤ 0.005), except for ash and hemicellulose. Regarding the season, the nutrient group matters greatly, but winter often determines significant differences (Table S5–S11). Gender only causes a significant difference in the ash content (p = 0.002). And the age class causes a significant difference in all nutrient groups (p ≤ 0.021) except ash and NFC. In most cases, the adult and juvenile animals do not differ significantly. Significant differences were mainly found between subadults and the two other age classes.
Average crude nutrient contents [%/DM] in roe deer rumen content from different habitats. Yellowish shades in the habitat legend represent agricultural habitats, greenish-brownish different forest communities and grey alpine habitats. (for further details, see Table 1). Nutrient levels were determined using Weender and VanSoest analysis. The crude protein content is composed of plant and microbial protein. NFC stands for non-fibre-carbohydrates, which mainly includes sugars and starches and pectins
Fermentation Products
Ammonia (p < 0.001) and lactate (p < 0.001) levels differ significantly between habitats (Fig. 2, Table S4). The significant differences are mainly due to the low ammonia content in habitats SF and AMF and the high content in habitats ABF and AG. The lactate content is significantly lower, especially in habitats BF and AMF and highest in habitat ABF. Gender and age class do not show any significant difference. However, the ammonia content differs significantly between the seasons (p < 0.001), and the lactate content does not (Table S5–S11).
There is a significant difference regarding the factor habitat for all VFAs (p < 0.01). This is mainly due to low levels of acetic acid in habitat SF, propionic acid in habitat BF, and butyric, isobutyric, valeric, and isovaleric acid in forest habitats BF, PF, and SF (Fig. 3, Table S4). Significantly high concentrations of acetic acid are found in ABF and AMF habitats, propionic acid in ABF and AG, and butyric and valeric acid mainly in the alpine habitats AMF and GSF. Isobutyric and isovaleric acids are highest in ABF, AG, and GSF habitats. There is also a significant difference between the age classes (p ≤ 0.03), except propionic acid, and the seasons (p < 0.001) (Table S5–S11). The significant differences between the seasons are mainly due to low values in winter for all VFAs. Gender, in turn, does not cause a significant difference in the content of VFAs.
Habitat Portraits
Habitat AG (Table S4, S5)
The crude nutrients in the rumen content had a low total fibre content. The available, total fibre content was mainly characterised by a high amount of hemicellulose (16.5%), whereas cellulose with 17% and lignin with 12% are below the average. The NFC content was medium at 14.3%, and the crude protein content was the highest of all habitats (26.8%, closely followed by habitat SF). Significant seasonal fluctuations occurred for lignin and NFC. The lignin content was highest in spring (13.8%) and winter (13.8%) and lowest in autumn (9.9%). Antagonistically, the NFC content was the highest in autumn and the lowest in spring. The protein content was highest in spring at 31% and lowest in winter at 23.7%. Ammonia and lactate concentrations have also their lowest point in winter. Ammonia increased again significantly in spring, up to 24.5 mM, while lactate was still low and reached its maximum values in summer and autumn (around 7.7 mM). Propionic, butyric, and valeric acids showed similar patterns throughout the year, with the highest concentration in autumn and the lowest in spring.
Habitat ABF (Table S4, S6)
Overall, there was a very low total fibre content in the rumen content (46% NDF), with a peak in summer (contrary to the other habitats) and with low amounts of lignin (12.3%). Crude protein (23%) and NFC (16.3%) content were within the Bavarian average. Significant seasonal variations occurred only for the NFC content with the highest amounts in autumn (18.1%), when more tree fruits, such as acorns and beechnuts, were consumed. The average lactate concentration (6.35 mM) and propionic acid (25.4 mM) were above the average of all investigated habitats. Ammonia concentration was the highest of all habitats, with 25.2 mM. Iso-butyric acid (0.69 mM) and iso-valeric acid (1.18 mM) were also significantly enhanced compared to other habitats.
Habitat BF (Table S4, S7)
The total fibre content in the rumen was relatively high with 50.2%; NFC (14.6%) and crude protein (22.7%) were in the medium range compared to other habitats. We found significant seasonal differences in fibres (especially hemicellulose), proteins, and NFCs. Crude protein and NFC increased significantly during this season. Lactate (1.92 mM), propionic (19.2 mM), butyric (9.7 mM), and isovaleric acid (0.5 mM) occur in very low concentrations.
Habitat PF (Table S4, S8)
The rumen content had significantly high total fibre contents (54.5% NDF), and all fibre fractions had their highest proportions in this habitat. The NFC content is only 13.5%, and the crude protein content was significantly low and the lowest of all habitats, with 19.8% on average. The proportion of valeric acid was also very low, with only 1.46 mM. Strong seasonal fluctuations occurred, especially in the fibre content (cellulose and lignin) and crude protein, as well as in some fermentation products. NDF, cellulose, and lignin reached their maximum value in winter and their lowest value in summer. The course of the crude protein content is antagonistic to this. Hemicellulose content was highest in spring.
Habitat SF (Table S4, S9)
The total fibre content in the rumen was relatively high, with 51% NDF, with comparatively few celluloses and more hemicellulose. The crude protein content was nearly the highest with 26.78%, just behind habitat AG, and the NFC content was the lowest of all habitats with 7.9% (peak in autumn with 9.4%). The course of crude protein and total fibre content were antagonistic, with the highest content of total fibre in winter and the lowest in summer. The proportion of cellulose (17.5%) was comparatively low compared to other habitats, and the proportion of hemicellulose (16.8%) was high. The curves of the fibre fractions were similar throughout the year, except for summer. While hemicellulose and lignin reached their lowest value here, the cellulose fraction was high in this season. In addition, the concentration of ammonia was the lowest at 13.9 mM. Significant seasonal differences were only found for total lipids and some fermentation products.
Habitat GSF (Table S4, S10)
The total fibre (46% NDF) and crude protein content (23%) was slightly below average (∅ 23.6 and 48%). On the other hand, the NFC content was high, with nearly 18%. Regarding the fibre fractions, the hemicellulose content was the lowest of all habitats (10.5%). In contrast, the lignin (15.1%) and the cellulose content (20.4%) were quite high. The total fibre content (NDF), as well as all fibre fractions, had their peak values in winter. Cellulose had a second peak in summer. The crude protein content was highest in spring. Moreover, the butyric acid (15.4 mM) and the valeric acid content (2.7 mM) were the highest of all habitats. Seasonal variations occurred mainly in protein and total fibre content, as well as in some fatty acids.
Habitat AMF (Table S4, S11)
The proportion of total fibres was low (44.3% NDF); NDF and all fibre fractions had the highest content in winter. The crude protein content was medium (23.3%), with a peak in spring, and the NFC content was highest in this habitat (18.6%), with the highest proportions in summer and autumn. Furthermore, the concentration of butyric acid (15.1 mM) and valeric acid (2.6 mM) was high, and the concentration of ammonia (13.8 mM) and lactate (3.3 mM) was relatively low. The typical strong seasonal fluctuations in the alpine region were also reflected in the distribution of nutrients (Fig. 5). Except for NFC and hemicellulose, all groups have significant seasonal differences.
Variation in the Composition of the Rumen Microbiota
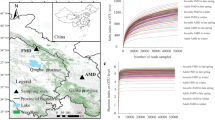
The bacterial microbiota in the rumen content of roe deer differed significantly in terms of habitat (p < 0.01), season (p < 0.01), and age class (p < 0.01, adult vs subadult vs juvenile). Most significant differences exist between the juvenile and subadult age classes. Gender was not causing a significant difference. The principal coordinate analysis (PCO) (Fig. 4A) showed the clustering of samples from habitats SF, GSF, AMF, and BF on the right side of the plot. The smallest distance is between habitat GSF and AMF. The second cluster of similarity is formed among habitat AG, ABF, and PF on the left side of the plot. Alpha-diversity analyses (Fig. 4B) showed that habitat GSF has the highest ruminal microbial Chao1 and Shannon index and habitat AG has the lowest. A Wilcoxon rank-sum test revealed significant differences between several pairwise results (see Table S1).
Heatmap of the significant associations (-log(qval)*sign(coeff)) between the five members of the core microbiota and the crude nutrients and fermentation products. Positive or negative signs indicate an enrichment or decrease in the corresponding genera in parallel with increasing nutrient contents and fermentation products. The analysis was performed with the MaAsLin2 R package
On average, Firmicutes (Bacillota) is the most frequent phylum in the roe deer rumen content (61%), followed by Bacteroidota (19%) and Actinobacteriota (14%) (Table S12).
Two hundred seventy-seven genera could be identified, from which 163 genera were detected in the rumen contents of all habitats (Fig. 5, Fig. S13). The remaining 114 genera were only found in some habitats and a few only in one of them (Fig. S2). Fifty-four of 277 genera could not be classified at the genus level but at higher taxonomic levels, showing the high number of still unknown genera in the roe deer rumen content.
Overall, the Christensenellaceae (R-7) is the most common genus with an average relative abundance of 20%, and it, therefore, is also the leading contributor to the, so-called core microbiota [35]. The common core microbiota of our roe deer consists of 5 genera, forming 44% of the total microbiota (Table S13). The identified genera are Eggerthellaceae (DNF00809), Prevotella, Christensenellaceae (R-7), Unclassified Lachnospiraceae, and Oscillospiraceae (NK4A214).
Significant differences between habitats can be found for most genera (Table S14). Christensenellaceae (R-7), as some representative of the core microbiota, shows no significant difference between the habitats. The significant difference for the genus Prevotella is due to the low values in the beech forest habitat (BF), whereas Oscillospiraceae (NK4A214) is more common there. In the agricultural habitat (AG), on the other hand, they showed a significantly low abundance. Eggerthellaceae (DNF00809) occur significantly less in agricultural-dominated areas and have their highest values in the pine (PF) and alpine mountain forest habitat (AMF). In the two alpine habitats (GSF and AMF), the uncl. Lachnospiraceae occur in significantly high abundances.
In addition to the core microbiota, some other genera, such as Fretibacterium, Latilactobacillus, Syntrophococcus, Streptococcus, Lentilactobacillus, Ralstonia, Tyzzerella, Catenisphaera, Enterococcus, and Leuconostoc, only occur in 1–2 habitats with increased abundance. Alternatively, Quinella or Treponema show a strikingly low abundance in one habitat (BF).
A MaAsLin2 analysis showed significant associations between the top representatives of the microbiota and some crude nutrients (CP, NDF, NFC) and fermentation products (Fig. 6).
Discussion
The present study investigated 311 rumen contents and the microbiota of wild roe deer living in seven different habitats across different seasons, animal ages, and gender. Especially based on a large number of samples, this study provides many more insights about wild ruminants and their microbiome as the number of studies is limited in contrast to domesticated ruminants. To the best of the author’s knowledge, only four publications studied European roe deer using amplicon sequencing methods [19, 36,37,38], and one the Chinese roe deer [39]. These studies mainly lack a representative number of rumen samples varying between 3 and 19. Studies with larger numbers of samples from deer were only carried out with faecal samples (red deer, 136 faecal samples) [40].
The challenge with relatively unexplored microbiomes is a large number of unclassified species. Previous studies in wild animals found a considerable number of unclassified OTUs [19, 35, 41, 42], as observed in the present study. Similarities were found at the phylum level between the present and previous studies focused on the roe deer ruminal microbiome by Ricci et al. [19] and Wilson et al. [37] and also in the rumen content of other wild ruminants, such as reindeer, elk, bison, moose, red deer, and sika deer [40, 42,43,44,45,46,47,48]. Firmicutes (Bacillota) was the dominant phylum, followed by Bacteroidota.
The rumen microbial ecosystem is dominated by a core community comprising Prevotella, unclassified Clostridiales, unclassified Bacteroidales, unclassified Ruminococcaceae, unclassified Lachnospiraceae, Ruminococcus, and Butyrivibrio, known as the “core microbiome”, which is found in domesticated and wild ruminants around the world [35, 41]. All these genera are present in the samples, but an exact comparison of the genera is not possible due to a deeper taxonomy classification. The core microbiota of the current Bavarian roe deer study contains five genera. The most frequently represented genus in the present samples is Christensenellaceae (R-7). In the worldwide comparison, Prevotella is in the lead, which is the second most common species in the roe deer samples.
The observed differences in microbial composition due to various habitats and the apparent impact of the forage were also studied for other wild ruminants, including reindeer and moose [42, 43], but mostly selectively composed diets were used [19, 49]. A high proportion of concentrate feed often characterises these. In other publications, natural forage was compared with selectively composed diets in the husbandry of actual wild ruminants [19, 40, 45, 47, 48, 50, 51].
The significant difference between the age classes can be explained by the animals eating different forages at different stages of development. It should be noted, however, that the data on juveniles are only available from September to April due to hunting seasons. Seasonal effects are caused by the sometimes strongly differing forage availability per season and were already analysed for Sika and White-tailed deer [52, 53].
In this study, we compared roe deer from seven different habitat types, representing Bavaria’s most characteristic and extreme habitats. The analysis of the crude nutrient and the plant species composition in the rumen contents showed that the roe deer are confronted with very different forage offers. This is also reflected in the ruminal microbiota. The average cross-habitat crude protein content is 23.6%. This is slightly lower than in the previous study (27.4%), in which only two habitats were investigated [3]. It must be considered that a large part of this is microbial protein and that vegetable protein is quickly converted into ammonia. A confirmation of that is that the ingested plants have significantly lower protein contents (coniferous trees: ~12.9%/DM; shrubs: ~17.3%/DM; herbs: ~19.4%/DM; grasses: ~3–12.6%/DM [54, 55]). Furthermore, nitrogen can be recovered via the ruminal-hepatic cycle. The average NFC content of 14.9% was higher than in the previous study (11.1%). As expected, the average fibre content (47.9% NDF, 27.8% CF) was high and corresponded to the values from the preliminary study (47.6% NDF, 27.8% CF). The protein content in the rumen content always reaches its minimum, and the total fibre content is at its maximum in winter (with the only exception: habitat ABF), regardless of how large the respective proportions are in the habitat as a whole. However, there are big differences between the individual fibre fractions.
Thanks to recent findings and the switch from the rigid classification into feeding categories, it is known that roe deer are pretty tolerant of high fibre content. This does not only apply to so-called intermediate types or roughage eaters [3, 14]. However, there are many differences between the seven habitats, some of which are significant, and each habitat has its own specific browsing range and nutrient profile. Commonalities, as demonstrated in Fig. 4, were found among all forest habitats (beech, spruce, grassland spruce and mountain forest), which are dominated by similar tree species, as well as shrub and dwarf shrub communities. Furthermore, the agricultural and agricultural beech habitats show strong similarities in cultivating the agricultural areas and the field edge vegetation. The pine habitat forms more of a cluster with the two agricultural habitats, which may indicate a tendency toward monoculture. The pine stands here are almost pure, and the ground vegetation is relatively poor in species. Likewise, the plant culture in the agricultural area is very one-sided in larger sections. Further correlations can be seen in the Shannon diversity index (Fig. 4 B), which is higher in the alpine habitats GSF and AMF, which could indicate adaptation to higher altitude habitats. Inhabitants of high altitudes have been described as having a more diverse microbiome than inhabitants of lowlands [56].
Interrelationships can also be depicted at the functional level. Functional relationships between microbial abundance in the rumen content and nutrient supply can be identified in several habitats. A correlation between nutrients, fermentation products, and the core microbiota members was shown for individual genera. Still, not all of them can be explained based on the literature. The genus Prevotella, for example, shows a significant decrease in association with increasing acetic acid, which is fibre-associated, whereas Prevotella is clearly protein-associated. The significant decrease in Oscillospiraceae (NK4A214) associated with increasing propionic acid results from cellulolytic properties [57], mainly increasing acetic acid production. Christensenellaceae (R-7) is also described in the literature as an acetic acid producer and fibre utiliser [57], so the positive correlation with propionic acid cannot be explained or must be influenced by unknown factors. The ammonia found in rumen contents is primarily the product of the breakdown of vegetable protein. A negative correlation with mainly fibre-associated species, like Christensenellaceae (R-7), Oscillospiraceae (NK4A214), and unclassified Lachnospiraceae [58], can therefore be explained.
The Eggerthellaceae (DNF00809), the core microbiota’s last representative, are significantly increasing with increasing NFC and NDF content. The Eggerthellaceae are known for degrading polyphenols [59], which could explain the association with fibre content. Another known function of the Eggerthellaceae is their involvement in maintaining homeostasis [60], which could explain a link with NFC content.
Across all habitats, a very differentiated, habitat-specific composition of the microbiota can be identified. Even four of the five taxa of the core microbiota show significant differences between the habitats. Not all habitat-specific distributions can be explained, as there are still many unclassified genera in the microbiome of wild ruminants whose functional assignment is unclear. However, some clear correlations can be shown between the available forage, the nutrients thus consumed, and the resulting microbiota.
The agriculture habitat (AG) was characterised by high protein availability, and hemicellulose dominated the fibre fractions. Hemicellulose is found in large amounts in field crops and sweet grasses, an essential part of the forage in this habitat. The lignin content was highest in winter and spring when many woody branches and shrubs were eaten. In autumn, on the other hand, the NFC content dominates due to tree fruits, which are increasingly found in the tree communities at the edges of the fields [6]. Accordingly, proteolytic genera such as Prevotella, Prevotellaceae (UCG001) [61], and Family XIII AD 3011group [62] play an important role here. Prevotella also has hemicellulolytic properties [61]. In contrast, Ruminococcus, Oscillospiraceae, and Clostridia are specialised in fibre utilisation [63, 64], especially cellulose [58], were present in significantly low numbers. Tyzzerella is described as a pathogenic bacterium [65] and occurs more frequently in spring. From winter to spring, the microbiota in the habitat AG changes very strongly (see Fig. S3). The increased occurrence of Tyzzerella at this time could possibly be connected to a microbial imbalance.
The Agriculture-Beech-Forest habitat (ABF) included large areas of agricultural land but also beech and oak-hornbeam forests [66]. The total fibre content in the rumen contents was relatively low. Protein and NFC content were in the mid-range. The NFC content was highest in autumn when the deer could browse the numerous beech and oak tree fruits [66]. These were available in large quantities, especially in the sample year 2018, as this was a fattening year for both species. The particularly high levels of ammonia, lactate, and some VFAs were also striking. This could explain the importance of the Ralstonia genus. Ralstonia is known to utilise VFAs and is capable of denitrification under anaerobic conditions [67, 68]. An N surplus in the course of the harvests could be an explanation for particularly high Ralstonia proportions in autumn. The proteolytic and pectinolytic genera Prevotella, Prevotellaceae (UCG 001), and Prevotellaceae (UCG 003) are also found in significant abundance in this agricultural habitat. As an important cellulolytic genus, Treponema [69] occurs in significantly high abundances in this habitat.
Large areas of pure beech forest are also located north of Bavaria, as in habitat beech forest (BF) [70, 71]. The rumen content contained significantly more total fibres with a high cellulose content. The protein content was in the medium range, and the NFC content was slightly below average. The seasonal fluctuations in this habitat were strongly reflected in the crude nutrient profile. A large proportion of shrubs (mainly) in the rumen contents dominated almost at all times of the year; only in spring do the conifers predominate, when presumably only a few deciduous shrubs are still to be found. Accordingly, high total fibre contents are found in the rumen content in winter and spring. Appropriately, the proportion of uncultured Lachnospiraceae and Enterococcus, which have fibrolytic properties [58, 72], occurs in significantly high abundances. In summer, tree and shrub fruits and herbs are added, which explains the crude protein and NFC peak in summer. The NFC content remains relatively high even in autumn when abundant beechnuts are available to the animals. The low proportion of Prevotella, Prevotellaceae (UCG 001), and Prevotellaceae (UCG 003) can be explained by the overall relatively low crude protein content and higher Olsenella (glucose fermenters) and Latilactobacillus (saccharolytic) proportions [73, 74], possibly by the higher sugar content in the forage in the summer months.
Another extreme habitat is the almost pure pine forests (PF) in the Upper Palatinate Basin [75, 76]. Accordingly, strong seasonal fluctuations in the nutrient profile are also reflected here. In this habitat, the deer mainly use dwarf shrubs, especially blueberry. Tree and field fruits, berries, cherries and apples are also on the otherwise very fibrous menu, depending on the season. The crude nutrient profile shows significantly high total fibre values, which, as expected, are highest in winter and lowest in summer. Again, the crude protein content is highest in summer, when shrubs, trees, and field crops supplement the forage. A clear connection can be drawn between the high proportions of Ruminococcus, which show their highest values in winter, parallel to the fibre values. The increased starch content of crops such as maize can explain the high proportion of Streptococci in summer. For the Eggerthellaceae (DNF00809), it is known that an essential function of genera belonging to this family is the maintenance or support of homeostasis [60], and they are increased by stress exposure. Since this genus is also more abundant in summer, this could be related to the forage’s significantly higher protein and starch content in summer. Genus Syntrophococcus is another fascinating bacteria with the highest abundance in habitat PF (1.15%). These bacteria can demethylate lignin [77]. The breakdown of lignin is otherwise only known from anaerobic fungi but not from rumen bacteria.
In contrast, the spruce forest habitat SF in the south of Munich [78] has high crude protein content in the nutrient profile. The total fibre content is also high over the year, with a high proportion of hemicellulose and lower cellulose content. Especially in winter, the animals must resort to coniferous wood, while herbs strongly dominate the forage composition the rest of the year. This explains the high protein content, which peaks in summer. In addition to deciduous wood and shrubs, the forage is strongly supplemented by forbs, cryptogams in autumn, and field crops in summer. The high protein content probably also explains the high proportion of proteolytic Prevotella, Prevotellaceae (UCG 001), Prevotellaceae (UCG 003), Butyrivibrio, and Eubacterium nodatum group [61, 79,80,81]. Most of them can also utilise hemicelluloses well [61, 80].
Characteristic Bavarian habitats are the alpine areas, which roe deer use up to a certain altitude. An important pre-alpine area is grassland farming, which is complemented by forest areas [82]. The Grassland-Spruce-Forest habitat (GSF) has a high NFC content in the nutrient profile. The total fibre and crude protein content are average. The total fibre content is highest in winter and lowest in spring. The lignin content is comparatively high, as is the cellulose content. In contrast, the crude protein content is highest in spring. The high protein content in spring can probably also be explained by the increased browsing of herbs in this habitat. However, the correlation between the botanical rumen content analysis (BRCA) and the crude nutrient profile must be considered with reservations, as only 13 samples from the BRCA were examined in this habitat. Moreover, these were mainly from the winter months, which also explains the relatively high proportion of anthropogenic bait feed in the form of apple pomace in the rumen contents. Significantly high abundances of cellulolytic unclassified Lachnospiraceae, Treponema, and Eubacterium hallii group can be explained by the high cellulose contents [58, 69, 79]. The saccharolytic genus Marvinbryantia [69] also has significantly high proportions, which fits with the high NFC values.
The Alpine Mountain Forest habitat (AMF) is in the middle of the mixed mountain forest of the Ruhpolding Limestone Alps [83]. Due to the alpine location, the animals living here must be able to adapt quickly to changing weather conditions and endure distinct seasons. The NFC content is also high in this alpine habitat; in fact, the highest proportions of all are found here. The total fibre content is relatively low, and the crude protein content is average. The total fibre content is only high in winter and low the rest of the year. The fractions are dominated by cellulose and lignin. The crude protein content is highest in spring, as in Habitat GSF. The NFC content is consistently high. Shrubs also dominate the food choice in this habitat, but the selection of shrubs consumed is much more diverse than, e.g. in habitat BF or PF. In summer and autumn, fruits also play a major role. A significantly increased proportion of Marvinbryantia is probably due to a starch- or sugar-associated forage. High proportions of Eggerthellaceae (DNF00809) and Enterorhabdus could be related to presumably high proportions of plant secondary metabolites in the pronounced herbaceous layer and various woody branched plants [84]. However, exact proportions have not yet been examined but will be part of the following analyses. Significantly occurring fibre-associated genera in this habitat are unclassified Clostridia, unclassified Lachnospiraceae, and Prevotellaceae (UCG 003).
In order to understand the needs or adaptive mechanisms of roe deer, this should be considered in the context of their surrounding habitat. The roe deer is highly and rapidly adaptable to the changing forage availability, contributing to these animals’ distribution success. However, they are also very well adapted to their habitat to make the best possible use of the given forage, even in months of privation. As we could already show in our preliminary study based on the energy density in the habitats, roe deer cope well with the given forage offer and adapt to less energy-rich food, e.g. by an increased rumen filling [4]. The energy densities from all the habitats studied are still being evaluated. Still, the animals’ weights already indicated no malnutrition or insufficient supply in any of the habitats. And results of the actual study show that the energy densities and the animals’ weights indicated no malnutrition or insufficient supply in any of the habitats [85]. For example, if we consider additional feeding in winter, habitat-specific adaptations should definitely be considered.
The dynamic changes in the microbiome structure support the colonisation of new habitats. Adaptation to a particular forage per se is, of course, also determined by their anatomy and by the environment in which they live, but we can see that the roe deer is a tolerant animal with strong dietary deviations involving plant species high in nutrients. The limits of this research were that due to the new nature of the study, several bacterial species have not yet been cultured; therefore, there were a considerable number of unclassified/unknown taxa among the sequencing data. However, this study opens the possibility of understanding the interplay between microbiome, crude nutrients, and VFAs. A future perspective will be to culture anaerobic species from the rumen of wild animals to better classify them.
Data Availability
The microbiota dataset analysed during the current study is available in the ENA repository, no. PRJEB61103.
References
Cederlund G (1983) Home range dynamics and habitat selection by roe deer in a boreal area in central Sweden. Acta Theriol 28(20):443–460
Wölfel H (2005) Biologie des Rehwildes und Konsequenzen für die jagdliche Praxis. oder: Das Reh ist kein Ungeziefer und der Jäger kein Schädlingsbekämpfer. Österreichische Jäger Tagung. Raumberg-Gumpenstein
Dahl S-A, Hudler M, Windisch W, Bolduan C, Brugger D, König A (2020) High fibre selection by roe deer (Capreolus capreolus): evidence of ruminal microbiome adaption to seasonal and geographical differences in nutrient composition. Anim Prod Sci. https://doi.org/10.1071/AN19376
König A, Hudler M, Dahl S-A, Bolduan C, Brugger D, Windisch W (2020) Response of roe deer (Capreolus capreolus) to seasonal and local changes in dietary energy content and quality. Anim Prod Sci. https://doi.org/10.1071/AN19375
Rahnenführer F (2022) Rehernährung in typischen bayerischen Habitattypen. Vergleich von Angebot und Aufnahme der gegebenen Äsung. Technical University of Munich, Freising
König A, Scheingraber M, Mitschke J (2016) Energiegehalt und Qualität der Nahrung von Rehen (Capreolus capreolus) im Jahresverlauf in zwei unterschiedlich geprägten Habitaten. Forstliche Forschungsberichte. Vol. 215. Zentrum Wald-Forst-Holz, Freising
Owens FN, Basalan M (2016) Ruminal Fermentation. In: Millen DD, De Beni Arrigoni M, Lauritano Pacheco RD (eds) Rumenology. Springer International Publishing, Switzerland, pp 63–102
Krause DO, Denman SE, Mackie RI, Morrison M, Rae AL, Attwood GT, McSweeney CS (2003) Opportunities to improve fiber degradation in the rumen: microbiology, ecology, and genomics. FEMS Microbiol Rev 27(5):663–693. https://doi.org/10.1016/s0168-6445(03)00072-x
Dehority BA (2003) Rumen Microbiology. Nottingham University Press, Nottingham
Anke M, Dittrich G, Groppel B, Schäfer U, Müller R, Hoppe C (2007) Zusammensetzung und Aufnahme von Winteräsung durch Reh-, Muffel-, Dam- und Rotwild. In: Stubbe M (ed) Beiträge zur Jagd- und Wildforschung. Gesellschaft für Wildtier- und Jagdforschung e.V., Halle, pp 379–398
Hofmann RR (1989) Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestives system. Oecologia 78(4):443–457. https://doi.org/10.1007/BF00378733
Bubenik A, Lochman J (1956) Futterverbrauch und Tagesrhythmus der Futteraufnahme bei Reh-und Rotwild. Z Jagdwiss 2:112–118
Hofmann RR (1982) Die Verdauungsorgane des Rehes und ihre Anpassung an die besondere Ernährungsweise. In: Hofmann RR (ed) Wildbiologische Informationen für den Jäger. Ferdinand Enke Verlag, Stuttgart, pp 103–126
Pérez-Barbería FJ, Elston DA, Gordon IJ, Illius AW (2004) The evolution of phylogenetic differences in the efficiency of digestion in ruminants. Proc Biol Sci 271(1543):1081–1090. https://doi.org/10.1098/rspb.2004.2714
Belanche A, Doreau M, Edwards JE, Moorby JM, Pinloche E, Newbold CJ (2012) Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation. J Nutr 142(9):1684–1692. https://doi.org/10.3945/jn.112.159574
Snelling TJ et al (2019) Temporal stability of the rumen microbiota in beef cattle, and response to diet and supplements. Animal Microbiome 1(1):16. https://doi.org/10.1186/s42523-019-0018-y
Seddik H, Xu L, Wang Y, Mao SY (2019) A rapid shift to high-grain diet results in dynamic changes in rumen epimural microbiome in sheep. Animal 13(8):1614–1622. https://doi.org/10.1017/S1751731118003269
Ishaq SL, Wright A-DG (2015) Wild Ruminants. In: Puniya AK, Singh R, Kamra DN (eds) Rumen microbiology: from evolution to revolution. Springer, India, pp 37–46
Ricci S, Sandfort R, Pinior B, Mann E, Wetzels SU, Stalder G (2019) Impact of supplemental winter feeding on ruminal microbiota of roe deer Capreolus capreolus. Wildl Biol 2019(1):1–11. https://doi.org/10.2981/wlb.00572
Mitchell B (1967) Growth layers in dental cement for determining the age of roe deer (Cervus elaphus L.). J Anim Ecol 36(2):279–293 https://doi.org/10.2307/2912
VDLUFA (2012) VDLUFA-methods book 3: the chemical analysis of feedstuffs. VDLUFA-Verlag, Darmstadt, Germany
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet journal 17(1):3. https://doi.org/10.14806/ej.17.1.200
Kaewtapee C et al (2017) Effect of Bacillus subtilis and Bacillus licheniformis supplementation in diets with low- and high-protein content on ileal crude protein and amino acid digestibility and intestinal microbiota composition of growing pigs. J Anim Sci Biotechnol 8(1):37. https://doi.org/10.1186/s40104-017-0168-2
Bolyen E et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37(8):852–857. https://doi.org/10.1038/s41587-019-0209-9
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13(7):581–583. https://doi.org/10.1038/nmeth.3869
Quast C et al (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. https://doi.org/10.1093/nar/gks1219
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30(4):772–780. https://doi.org/10.1093/molbev/mst010
Price MN, Dehal PS, Arkin AP (2010) FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS One 5(3):e9490. https://doi.org/10.1371/journal.pone.0009490
R Core Team Ro (2013) R: A language and environment for statistical computing
McMurdie PJ, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8(4):e61217. https://doi.org/10.1371/journal.pone.0061217
Dixon P (2003) VEGAN, a package of R functions for community ecology. J Veg Sci 14(6):927–930. https://doi.org/10.1111/j.1654-1103.2003.tb02228.x
Wickham H (2016) ggplot2: elegant graphics for data analysis. In: Wickham H (ed). Springer International Publishing, Cham
Conway JR, Lex A, Gehlenborg N (2017) UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics. 33(18):2938–2940
Mallick H et al (2021) Multivariable association discovery in population-scale meta-omics studies. PLoS Comput Biol 17(11):e1009442
Henderson G, Cox F, Ganesh S, Jonker A, Young W, Collaborators GRC, Janssen PH (2015) Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep 5:1–13. https://doi.org/10.1038/srep14567
Ostbye K, Wilson R, Rudi K (2016) Rumen microbiota for wild boreal cervids living in the same habitat. FEMS Microbiol Lett 363(20):1–6. https://doi.org/10.1093/femsle/fnw233
Wilson R, Ostbye K, Angell IL, Rudi K (2019) Association between diet and rumen microbiota in wild roe deer. FEMS Microbiol Lett 366(6):1–5. https://doi.org/10.1093/femsle/fnz060
Miltko R, Kowalik B, Majewska MP, Kedzierska A, McEwan NR, Belzecki G (2020) The effect of protozoa on the bacterial composition and hydrolytic activity of the roe deer rumen. Animals (Basel) 10(3). https://doi.org/10.3390/ani10030467
Li Z et al (2014) Bacteria and methanogens differ along the gastrointestinal tract of Chinese roe deer (Capreolus pygargus). PLoS One:1–20. https://doi.org/10.1371/journal.pone.0114513
Menke S, Heurich M, Henrich M, Wilhelm K, Sommer S (2019) Impact of winter enclosures on the gut bacterial microbiota of red deer in the Bavarian Forest National Park. Wildl Biol 2019(1). https://doi.org/10.2981/wlb.00503
Pope PB et al (2012) Metagenomics of the Svalbard reindeer rumen microbiome reveals abundance of polysaccharide utilization loci. PLoS One 7(6):e38571. https://doi.org/10.1371/journal.pone.0038571
Sundset MA, Praesteng KE, Cann IKO, Mathiesen SD, Mackie RI (2007) Novel rumen bacterial diversity in two geographically separated sub-species of reindeer. Microb Ecol 54:424–438. https://doi.org/10.1007/s00248-007-9254-x
Ishaq SL, Wright A-D (2014) High-throughput DNA sequencing of the ruminal bacteria from moose (Alces alces) in Vermont, Alaska, and Norway. Microb Ecol 68:185–195. https://doi.org/10.1007/s00248-014-0399-0
Laishev KA, Ilina LA, Filippova VA, Dunyashev TP, Laptev GY, Abakumov EV (2020) Rumen bacterial community of young and adult of reindeer (Rangifer tarandus) from Yamalo-Nenets Autonomous District of Russia. Open Agric 5:10–20. https://doi.org/10.1515/opag-2020-0001
Salgado-Flores A, Hagen LH, Ishaq SL, Zamanzadeh M, Wright A-D, Pope PB, Sundset MA (2016) Rumen and cecum microbiomes in reindeer (Rangifer tarandus tarandus) are changed in response to a lichen diet and may affect enteric methane emissions. PLoS One. https://doi.org/10.1371/journal.pone.0155213
Bergmann GT, Craine JM, Robeson II MS, Fierer N (2015) Seasonal shifts in diet and gut microbiota of the American bison (Bison bison). PLoS One. https://doi.org/10.1371/journal.pone.0142409
Couch CE et al (2021) Effects of supplemental feeding on the fecal bacterial communities of Rocky Mountain elk in the Greater Yellowstone Ecosystem. PLoS One. https://doi.org/10.1371/journal.pone.0249521
Guan Y, Yang H, Han S, Feng L, Wang T, Ge J (2017) Comparison of the gut microbiota composition between wild and captive sika deer (Cervus nippon hortulorum) from feces by high-throughput sequencing. AMB Express 7(212):1–13. https://doi.org/10.1186/s13568-017-0517-8
Li ZP et al (2013) Molecular diversity of rumen bacterial communities from tannin-rich and fiber-rich forage fed domestic Sika deer (Cervus nippon) in China. BMC Microbiol 13:1–12. https://doi.org/10.1186/1471-2180-13-151
Li Z, Wright A-DG, Liu H, Fan Z, Yang F, Zhang Z, Li G (2015) Response of the Rumen Microbiota of Sika Deer (Cervus nippon) Fed Different Concentrations of Tannin Rich Plants. PLoS One. https://doi.org/10.1371/journal.pone.012348
Minich D et al (2021) Alterations in gut microbiota linked to provenance, sex, and chronic wasting disease in white-tailed deer (Odocoileus virginianus). Sci Rep 11(13218):1–12. https://doi.org/10.1038/s41598-021-89896-9
Ichimura Y et al (2004) Rumen microbes and fermentation of wild sika deer on the Shiretoko peninsula of Hokkaido Island, Japan. Ecol Res 19:389–395. https://doi.org/10.1111/j.1440-1703.2004.00649.x
Delgado ML et al (2017) Intestinal microbial community dynamics of white-tailed deer (Odocoileus virginianus) in an agroecosystem. Microb Ecol 74:496–506. https://doi.org/10.1007/s00248-017-0961-7
Esser W (1958) Beitrag zur Untersuchung der Äsung des Rehwildes. Z Jagdwiss 4(1):1–40
Klötzli F (1965) Qualität und Quantität der Rehäsung. Eidgenössische Technische Hochschule Zürich, Zürich
Li B et al (2022) Rumen microbiota of indigenous and introduced ruminants and their adaptation to the Qinghai–Tibetan plateau. Front Microbiol 13:10.3389%2Ffmicb.2022.1027138
Morotomi M, Nagai F, Watanabe Y (2012) Description of Christensenella minuta gen. nov., sp. nov., isolated from human faeces, which forms a distinct branch in the order Clostridiales, and proposal of Christensenellaceae fam. nov. Int J Syst Evol Microbiol 62(1):144–149. https://doi.org/10.1099/ijs.0.026989-0
Biddle A, Stewart L, Blanchard J, Leschine S (2013) Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 5:627–640. https://doi.org/10.3390/d5030627
Rodríguez-Daza M-C et al (2020) Berry polyphenols and fibers modulate distinct microbial metabolic functions and gut microbiota enterotype-like clustering in obese mice. Front Microbiol 11. https://doi.org/10.3389/fmicb.2020.02032
Wang K et al (2022) Characterization of the microbial communities along the gastrointestinal tract in crossbred cattle. Animals. 12(825):1–12. https://doi.org/10.3390/ani12070825
Purushe J et al (2010) Comparative genome analysis of Prevotella ruminicola and Prevotella bryantii: insights into their environmental niche. Microb Ecol 60:721–729. https://doi.org/10.1007/s00248-010-9692-8
Shi J et al (2020) High-meat-protein high-fat diet induced dysbiosis of gut microbiota and tryptophan metabolism in Wistar rats. J Agric Food Chem 68(23):6333–6346. https://doi.org/10.1021/acs.jafc.0c00245
Yildirim E et al (2021) The structure and functional profile of ruminal microbiota in young and adult reindeers (Rangifer tarandus) consuming natural winter-spring and summer-autumn seasonal diets. PeerJ. 9:e12389. https://doi.org/10.7717/peerj.12389
Mu C, Zhang L, He X, Smidt H, Zhu W (2017) Dietary fibres modulate the composition and activity of butyrate-producing bacteria in the large intestine of suckling piglets. Antonie Van Leeuwenhoek 110(5):687–696. https://doi.org/10.1007/s10482-017-0836-4
Hao Y et al (2020) Effects of paper mulberry silage on the milk production, apparent digestibility, antioxidant capacity, and fecal bacteria composition in Holstein dairy cows. Animals. 10(7):1152
Lohr F (2015) Universitätsforstamt Sailershausen Operat. Bayerische Forstschule & Bayerische Technikerschule für Waldwirtschaft Lohr a.Main: Lohr a. Main
Schwartz E, Friedrich B (2001) A physical map of the megaplasmid pHG1, one of three genomic replicons in Ralstonia eutropha H16. FEMS Microbiol Lett 201:213–219. https://doi.org/10.1111/j.1574-6968.2001.tb10759.x
Chakraborty P, Gibbons W, Muthukumarappan K (2009) Conversion of volatile fatty acids into polyhydroxyalkanoate by Ralstonia eutropha. J Appl Microbiol 106:1996–2005. https://doi.org/10.1111/j.1365-2672.2009.04158.x
Dai Y et al (2016) The composition, localization and function of low-temperature-adapted microbial communities involved in methanogenic degradations of cellulose and chitin from Qinghai–Tibetan Plateau wetland soils. J Appl Microbiol 121(1):163–176. https://doi.org/10.1111/jam.13164
Bayerische Staatsforsten FR (2013) Naturschutzkonzept für den Forstbetrieb Rothenbuch. Bayerische Staatsforsten Rothenbuch, Deutschland
Bayerische Staatsforsten FH (2014) Naturschutzkonzept für den Forstbetrieb Heigenbrücken. Bayerische Staatsforsten: Heigenbrücken, Deutschland
Zhao J, Shao T, Chen S, Tao X, Li J (2021) Characterization and identification of cellulase-producing Enterococcus species isolated from Tibetan yak (Bos grunniens) rumen and their application in various forage silages. J Appl Microbiol 131(3):1102–1112. https://doi.org/10.1111/jam.15014
Kraatz M, Wallace RJ, Svensson L (2011) Olsenella umbonata sp. nov., a microaerotolerant anaerobic lactic acid bacterium from the sheep rumen and pig jejunum, and emended descriptions of Olsenella, Olsenella uli and Olsenella profusa. Int J Syst Evol Microbiol 61:795–803. https://doi.org/10.1099/ijs.0.022954-0
Guo X, Xu D, Li F, Bai J, Su R (2023) Current approaches on the roles of lactic acid bacteria in crop silage. Microb Biotechnol 16(1):67–87. https://doi.org/10.1111/1751-7915.14184
Bayerische Staatsforsten FR (2015) Naturschutzkonzept für den Forstbetrieb Roding. Bayerische Staatsforsten Roding, Deutschland
Bayerische Staatsforsten FB (2015) Regionales Naturschutzkonzept für den Forstbetrieb Burglengenfeld. Bayerische Staatsforsten Burglengenfeld, Deutschland
Mosoni P, Besle J-M, Toillon S, Jouany J-P (1994) Transformations of (C14-lignin) cell walls of wheat by rumen microorganisms. J Sci Food Agric 64:379–387. https://doi.org/10.1002/jsfa.2740640321
Bayerische Staatsforsten FM (2016) Naturschutzkonzept für den Forstbetrieb München. Bayerische Staatsforsten München, Deutschland
Wei Z, Xie X, Xue M, Valencak TG, Liu J, Sun H (2021) The effects of non-fiber carbohydrate content and forage type on rumen microbiome of dairy cows. Animals. 11(12):3519
Kopečný J, Zorec M, Mrázek J, Kobayashi Y, Marinšek-Logar R (2003) Butyrivibrio hungatei sp. nov. and Pseudobutyrivibrio xylanivorans sp. nov., butyrate-producing bacteria from the rumen. Int J Syst Evol Microbiol 53(1):201–209. https://doi.org/10.1099/ijs.0.02345-0
Cotta MA, Hespell RB (1986) Proteolytic activity of the ruminal bacterium Butyrivibrio fibrisolvens. Appl Environ Microbiol 52(1):51–58. https://doi.org/10.1128/aem.52.1.51-58.1986
Bayerische Staatsforsten FS (2014) Naturschutzkonzept für den Forstbetrieb Sonthofen. Bayerische Staastsforsten, Sonthofen
Bayerische Staatsforsten FR (2015) Naturschutzkonzept Forstbetrieb Ruhpolding. Bayerische Staatsforsten, Ruhpolding, Deutschland
Basu A, Lyons TJ (2012) Strawberries, blueberries, and cranberries in the metabolic syndrome: clinical perspectives. J Agric Food Chem 60(23):5687–5692. https://doi.org/10.1021/jf203488k
König A, Dahl S-A, Windisch W (2023) Energy intake and nutritional balance of roe deer (Capreolus capreolus) in special Bavarian landscapes in southern Germany. Anim Prod Sci. https://doi.org/10.1071/AN23034
Acknowledgements
We would like to thank the Bavarian State Ministry of Food, Agriculture and Forestry and the Academy for the Protection of Zoo Animals and Wildlife, e.V. for their financial support of the project and the Technical University of Munich for their support to publish the research results in open access. Furthermore, our sincere thanks go to the Burglengenfeld, Heigenbrücken, Munich, Roding, Rothenbuch, Ruhpolding, and Sonthofen operations of the Bavarian State Forests, the University Forestry Service Würzburg, as well as Mr Klaus Urban and Mr Kai Bomans for supporting the project on-site, especially for providing the sample material. We would also like to thank all the student assistants involved for their active support in the field and the laboratory, Nida Amin for her introduction to the statistical programmes and Beate Mezger for her intensive support in the laboratory analysis of the microbiota.
Funding
Open Access funding enabled and organized by Projekt DEAL. This project was financially supported by The Bavarian State Ministry of Food, Agriculture and Forestry and the Academy for the Protection of Zoo Animals and Wildlife, e.V. The Technical University of Munich funded the open-access publication.
Author information
Authors and Affiliations
Contributions
Sarah-Alica Dahl (S.-A. D.), Jana Seifert (J.S.), Amélia Camarinha Silva (A. C. S.) and Andreas König (A. K.) contributed to the study conception and design. Material preparation and data collection were performed by S.-A. D. and Martina Hudler. Data analysis was performed by S.-A. D., Yu-Chieh Cheng, Angélica Hernández-Arriaga, J.S. and A. C. S.. The first draft of the manuscript was written by S.-A. D., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Roe deer are in Germany a considered game species according to the Federal Hunting Act, paragraph 2, section 1 and are therefore ownerless. The roe deer samples were obtained in the course of regular hunting seasons and outside these. Samples taken outside the regular hunting season were based on a lifted closed season, according to Article 33 subparagraph 5 of the Bavarian Hunting Act in conjunction with § 22 subparagraph 1 sentence 3 of the Federal Hunting Act. District Council of Rottal am Inn, Fn: 31-7512-01/13 and Fn: 31-7512-02/13; District Council of Starnberg Fn: 7512/311.2W; District Council of Munich Fn: 5.3-750/Hei; District Council of Haßberge Fn: 1/2-751/1-1; District Council of Traunstein Fn: 5.351-7512-830/860; District Council of Aschaffenburg Fn: 41.3-7533; District Council of Oberallgäu Fn: LRA OA-7512-WE/Ze; District Council of Schwandorf Fn: 4.14-750/120329 and 4.14-750/120327.
Competing Interests
The authors declare no competing interests.
Supplementary Information
ESM 1:
(PDF 1145 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dahl, SA., Seifert, J., Camarinha-Silva, A. et al. Microbiota and Nutrient Portraits of European Roe Deer (Capreolus capreolus) Rumen Contents in Characteristic Southern German Habitats. Microb Ecol 86, 3082–3096 (2023). https://doi.org/10.1007/s00248-023-02308-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-023-02308-5