Abstract
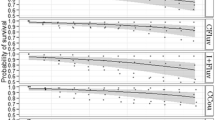
Large-scale honey bee colony losses reported around the world have been associated with intoxication with pesticides, as with the presence of pests and pathogens. Among pesticides, neonicotinoid insecticides are the biggest threat. Due to their extensive use, they can be found in all agricultural environments, including soil, water, and air, are persistent in the environment, and are highly toxic for honey bees. In addition, infection by different pests and pathogens can act synergistically, weakening bees. In this study, we investigated the effects of chronic exposure to sublethal doses of imidacloprid alone or combined with the microsporidia Nosema ceranae on the immune response, deformed wing virus infection (DWV), gut microbiota, and survival of Africanized honey bees. We found that imidacloprid affected the expression of some genes associated with immunity generating an altered physiological state, although it did not favor DWV or N. ceranae infection. The pesticide alone did not affect honey bee gut microbiota, as previously suggested, but when administered to N. ceranae infected bees, it generated significant changes. Finally, both stress factors caused high mortality rates. Those results illustrate the negative impact of imidacloprid alone or combined with N. ceranae on Africanized honey bees and are useful to understand colony losses in Latin America.




Similar content being viewed by others
Data Availability
The sequence datasets generated during the current study are available in the NCBI BioProject database (accession number PRJNA741666).
Code Availability
Not applicable.
References
Potts SG, Imperatriz-Fonseca V, Ngo HT, Aizen MA, Biesmeijer JC, Breeze TD, Dicks LV, Garibaldi LA, Hill R, Settele J, Vanbergen AJ (2016) Safeguarding pollinators and their values to human well-being. Nature 540:220–229. https://doi.org/10.1038/nature20588
Kulhanek K, Steinhauer N, Rennich K, Caron DM, Sagili RR, Pettis JS, Ellis JD, Wilson ME, Wilkes JT, Tarpy DR, Rose R, Lee K, Rangel J, vanEngelsdorp D (2017) A national survey of managed honey bee 2015–2016 annual colony losses in the USA. J Apic Res 56:328–340. https://doi.org/10.1080/00218839.2017.1344496
Requier F, Antúnez K, Morales CL, Aldea Sánchez P, Castilhos D, Garrido PM, Giacobino A, Reynaldi FJ, Rosso Londoño JM, Santos E, Garibaldi LA (2018) Trends in beekeeping and honey bee colony losses in Latin America. J Apic Res 57:657–662. https://doi.org/10.1080/00218839.2018.1494919
Gray A, Adjlane N, Arab A, Ballis A, Brusbardis V, Charrière JD, Chlebo R, Coffey MF, Cornelissen B, Amaro da Costa C, Dahle B, Danihlík J, Dražić MM, Evans G, Fedoriak M, Forsythe I, Gajda A, de Graaf DC, Gregorc A, Ilieva I, Johannesen J, Kauko L, Kristiansen P, Martikkala M, Martín-Hernández R, Medina-Flores CA, Mutinelli F, Patalano S, Raudmets A, San Martin G, Soroker V, Stevanovic J, Uzunov A, Vejsnaes F, Williams A, Zammit-Mangion M, Brodschneider R (2020) Honey bee colony winter loss rates for 35 countries participating in the COLOSS survey for winter 2018–2019, and the effects of a new queen on the risk of colony winter loss. J Apic Res 59:744–751. https://doi.org/10.1080/00218839.2020.1797272
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1435–1446. https://doi.org/10.1126/science.1255957
Sanchez-Bayo F, Goka K (2014) Pesticide residues and bees–a risk assessment. PLoS ONE 9(4):e94482. https://doi.org/10.1371/journal.pone.0094482
Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Furlan L, Gibbons DW, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke CH, Liess M, Long E, McField M, Mineau P, Mitchell EAD, Morrissey CA, Noome DA, Pisa L, Settele J, Stark JD, Tapparo A, Van Dyck H, Van Praagh J, Van der Sluijs JP, Whitehorn PR, Wiemers M (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res 22:5–34. https://doi.org/10.1007/s11356-014-3470-y
Jiang J, Ma D, Zou N, Yu X, Zhang Z, Liu F, Mu W (2018) Concentrations of imidacloprid and thiamethoxam in pollen, nectar and leaves from seed-dressed cotton crops and their potential risk to honeybees (Apis mellifera L.). Chemosphere 201:159–167. https://doi.org/10.1016/j.chemosphere.2018.02.168
Blacquiere T, Smagghe G, Van Gestel CA, Mommaerts V (2012) Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicology 21:973–992. https://doi.org/10.1007/s10646-012-0863-x
Bonmatin JM, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke C, Liess M, Long E, Marzaro M, Mitchell EAD, Noome DA, Simon-Delso N, Tapparo A (2015) Environmental fate and exposure; neonicotinoids and fipronil. Environ Sci Pollut Res 22(1):35–67. https://doi.org/10.1007/s11356-014-3332-7
Mitchell EA, Mulhauser B, Mulot M, Mutabazi A, Glauser G, Aebi A (2017) A worldwide survey of neonicotinoids in honey. Science 358(6359):109–111. https://doi.org/10.1126/science.aan3684
Lunardi JS, Zaluski R,Orsi RO (2017) Evaluation of motor changes and toxicity of insecticides fipronil and imidacloprid in Africanized honeybees (Hymenoptera: Apidae). Sociobiology 64: 50–56. https://doi.org/10.13102/sociobiology.v64i1.1190
Steinhauer N, Kulhanek K, Antúnez K, Human H, Chantawannakul P, Chauzat MP, vanEngelsdorp D (2018) Drivers of colony losses. Curr Opin Insect Sci 26:142–148. https://doi.org/10.1016/j.cois.2018.02.004
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103:S96–S119. https://doi.org/10.1016/j.jip.2009.07.016
Genersch E, Von Der Ohe W, Kaatz H, Schroeder A, Otten C, Büchler R, Berg S, Ritter W, Mühlen W, Gisder S, Meixner M, Rosenkranz LG, P, (2010) The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie 41(3):332–352. https://doi.org/10.1051/apido/2010014
Schroeder DC, Martin SJ (2012) Deformed wing virus: the main suspect in unexplained honeybee deaths worldwide. Virulence 3:589–591. https://doi.org/10.4161/viru.22219
Martin SJ, Brettell LE (2019) Deformed wing virus in honeybees and other insects. Annu Rev Virol 6:49–69. https://doi.org/10.1146/annurev-virology-092818-015700
Higes M, Martín Hernández R, Meana A (2010) Nosema ceranae in Europe: an emergent type C nosemosis. Apidologie 41:375–392. https://doi.org/10.1051/apido/2010019
Antúnez K, Martín-Hernández R, Prieto L, Meana A, Zunino P, Higes M (2009) Immune-suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ Microbiol 11:2284–2290. https://doi.org/10.1111/j.1462-2920.2009.01953.x
Evans JD, Spivak M (2010) Socialized medicine: individual and communal disease barriers in honeybees. J Invertebr Pathol 103:S62–S72. https://doi.org/10.1016/j.jip.2009.06.019
Doublet V, Poeschl Y, Gogol-Döring A, Alaux C, Annoscia D, Aurori C, Barribeau SM, Bedoya-Reina OC, Brown MJF, Bull JC, Flenniken ML, Galbraith DA, Genersch E, Gisder S, Grosse I, Holt HL, Hultmark D, Lattorff HMG, Le Conte Y, Manfredini F, McMahon DP, Moritz RFA, Nazzi F, Niño EL, Nowick K, van Rij RP, Paxton RJ, Grozinger CM (2017) Unity in defence: honeybee workers exhibit conserved molecular responses to diverse pathogens. BMC Genomics 18:207. https://doi.org/10.1186/s12864-017-3597-6
Wyatt GR (1961) The biochemistry of insect hemolymph. Annu Rev Entomol 6(1):75–102. https://doi.org/10.1146/annurev.en.06.010161.000451
Casteels P, Ampe C, Jacobs F, Tempst P (1993) Functional and chemical characterization of Hymenoptaecin, an antibacterial polypeptide that is infection-inducible in the honeybee (Apis mellifera). J Biol Chem 268:7044–7054. https://doi.org/10.1016/S0021-9258(18)53143-4
Schneider PM (1985) Purification and properties of three lysozymes from hemolymph of the cricket, Gryllus bimaculatus (De Geer). Insect Biochem 15(4):463–470. https://doi.org/10.1016/0020-1790(85)90058-7
White JW Jr, Subers MH, Schepartz AI (1963) The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim Biophys Acta 73(1):57–70. https://doi.org/10.1016/0926-6569(63)90108-1
Corona M, Velarde R, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, Robinson GE (2007) Vitellogenin, juvenile hormone, insulin signalling, and queen honey bee longevity. Proc Natl Acad Sci 104:7128–7133. https://doi.org/10.1073/pnas.0701909104
Salmela H, Amdam GV, Freitak D (2015) Transfer of immunity from mother to offspring is mediated via egg-yolk protein vitellogenin. PLoS Pathog 11(7):e1005015. https://doi.org/10.1371/journal.ppat.1005015
Brandt A, Gorenflo A, Siede R, Meixner M, Büchler R (2016) The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honeybees (Apis mellifera L.). J Insect Physiol 86:40–47. https://doi.org/10.1016/j.jinsphys.2016.01.001
Alaux C, Brunet JL, Dussaubat C, Mondet F, Tchamitchan S, Cousin M, Brillard J, Belzunces LP, Le Conte Y (2010) Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ Microbiol 12:774–782. https://doi.org/10.1111/j.1462-2920.2009.02123.x
Di Prisco G, Cavaliere V, Annoscia D, Varricchio P, Caprio E, Nazzi F, Gargiulo G, Pennacchio F (2013) Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honeybees. Proc Natl Acad Sci 110:18466–18471. https://doi.org/10.1073/pnas.1314923110
Kwong WK, Moran NA (2016) Gut microbial communities of social bees. Nat Rev Microbiol 14(6):374–384. https://doi.org/10.1038/nrmicro.2016.43
Raymann K, Moran NA (2018) The role of the gut microbiome in health and disease of adult honey bee workers. Curr Opin Insect Sci 26:97–104. https://doi.org/10.1016/j.cois.2018.02.012
Kwong WK, Moran NA (2013) Cultivation and characterization of the gut symbionts of honeybees and bumble bees: description of Snodgrassella alvi gen. nov., sp. nov., a member of the family Neisseriaceae of the Betaproteobacteria, and Gilliamella apicola gen. nov., sp. nov., a member of Orbaceae fam. nov., Orbales ord. nov., a sister taxon to the order ‘Enterobacteriales’ of the Gammaproteobacteria. Int J Syst 63(Pt_6): 2008–2018. https://doi.org/10.1099/ijs.0.044875-0
Ellengaard KM, Tamarit D, Javelind E, Olofsson TC, Andersson SG, Vásquez A (2015) Extensive intra-phylotype diversity on lactobacilli and bifidobacteria from the honeybee gut. BMC Genomics 16(1):1–22. https://doi.org/10.1186/s12864-015-1476-6
Engel P, Kwong WK, Moran NA (2013a) Frischella perrara gen. nov., sp. nov., a gammaproteobacterium isolated from the gut of the honeybee, Apis mellifera. Int J Syst Evol Microbiol 63(Pt_10):3646–3651. https://doi.org/10.1099/ijs.0.049569-0
Kešnerová L, Moritz R, Engel P (2016) Bartonella apis sp. nov., a honey bee gut symbiont of the class Alphaproteobacteria. Int J Syst Evol 66(1):414–421. https://doi.org/10.1099/ijsem.0.000736
Corby-Harris V, Snyder LA, Schwan MR, Maes P, McFrederick QS, Anderson KE (2014) Origin and effect of Alpha 2.2 Acetobacteraceae in honey bee larvae and description of Parasaccharibacter apium gen. nov., sp. nov. Appl Environ Microbiol 80:7460–7472. https://doi.org/10.1128/AEM.02043-14
Smith EA, Anderson KE, Corby-Harris V, McFrederick QS, Newton ILG (2021) Reclassification of seven honey bee symbiont strains as Bombella apis. Int J Syst Evol Microbiol 71(9):004950. https://doi.org/10.1099/ijsem.0.004950
Schwarz RS, Moran NA, Evans JD (2016) Early gut colonizers shape parasite susceptibility and microbiota composition in honey bee workers. Proc Natl Acad Sci 113(33):9345–9350. https://doi.org/10.1073/pnas.1606631113
Raymann K, Motta EV, Girard C, Riddington IM, Dinser JA, Moran NA (2018) Imidacloprid decreases honey bee survival rates but does not affect the gut microbiome. Appl Environ Microbiol 84:e00545-e618. https://doi.org/10.1128/AEM.00545-18
Rouzé R, Moné A, Delbac F, Belzunces L, Blot N (2019) The honeybee gut microbiota is altered after chronic exposure to different families of insecticides and infection by Nosema ceranae. Microbes Environ 34:226–233. https://doi.org/10.1264/jsme2.ME18169
Fries I, Chauzat MP, Chen YP, Doublet V, Genersch E, Gisder S, Higes M, McMahon DP, Martín-Hernández R, Natsopoulou M, Paxton RJ, Tanner G, Webster TC, Williams GR (2013) Standard methods for Nosema research. J Apic Res 52:1–28. https://doi.org/10.3896/IBRA.1.52.1.14
Martín-Hernández R, Meana A, Prieto L, Martinez SA, Garrido-Bailón E, Higes M (2007) Outcome of colonization of Apis mellifera by Nosema ceranae. Appl Environ Microbiol 73:6331–6338. https://doi.org/10.1128/AEM.00270-07
Bovi TS, Zaluski R, Orsi RO (2018) Toxicity and motor changes in Africanized honeybees (Apis mellifera L.) exposed to fipronil and imidacloprid. Anais Acad Brasil Ci 90:239–245. https://doi.org/10.1590/0001-3765201820150191
Castelli L, Balbuena S, Branchiccela B, Zunino P, Liberti J, Engel P, Antúnez K (2021) Impact of chronic exposure to sublethal doses of glyphosate on honey bee immunity, gut microbiota and infection by pathogens. Microorganisms 9:845. https://doi.org/10.3390/microorganisms9040845
Dietemann V, Nazzi F, Martin SJ, Anderson DL, Locke B, Delaplane KS, Wauquiez Q, Tannahill C, Frey E, Ziegelmann B, Rosenkranz P, Ellis JD (2013) Standard methods for varroa research. J Apic Res 52(1):1–54. https://doi.org/10.3896/IBRA.1.52.1.09
Williams GR, Alaux C, Costa C, Csaki T, Doublet V, Eisenhardt D, Fries I, Kuhn R, McMahon DP, Medrzycki P, Murray TE, Natsopoulou ME, Neumann P, Oliver R, Paxton RJ, Pernal SF, Shutler D, Tanner G, van der Steen JJM, Brodschneider R (2013) Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J Apic Res 52:1–36. https://doi.org/10.3896/IBRA.1.52.1.04
Kukielka D, Esperón F, Higes M, Sánchez-Vizcaíno JM (2008) A sensitive one-step real-time RT-PCR method for detection of deformed wing virus and black queen cell virus in honeybee Apis mellifera. J Virol Methods 147:275–281. https://doi.org/10.1016/j.jviromet.2007.09.008
Yang X, Cox-Foster DL (2005) Impact of an ectoparasite on the immunity and pathology of an invertebrate: evidence for host immunosuppression and viral amplification. PNAS 102(21):7470–7475. https://doi.org/10.1073/pnas.0501860102
Evans JD (2006) Beepath: an ordered quantitative-PCR array for exploring honey bee immunity and disease. J Invertebr Pathol 93:135–139. https://doi.org/10.1016/j.jip.2006.04.004
Engel P, James RR, Koga R, Kwong WK, McFrederick QS, Moran NA (2013) Standard methods for research on Apis mellifera gut symbionts. J Apic Res 52:1–24. https://doi.org/10.3896/IBRA.1.52.4.07
Zhou J, Bruns MA, Tiedje JM (1996) DNA recovery from soils of diverse composition. Appl Environ Microbiol 62:316–322. https://doi.org/10.1128/aem.62.2.316-322.1996
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:387–409. https://doi.org/10.1093/nar/29.9.e45
Xie F, Xiao P, Chen D, Xu L, Zhang B (2012) miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol Biol 80(1):75–84. https://doi.org/10.1007/s11103-012-9885-2
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodriguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu YX, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vazquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
RStudio Team (2020) RStudio: integrated development for R. Boston: RStudio, PBC. URL http://www.rstudio.com/
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17:10–12. https://doi.org/10.14806/ej.17.1.200
McMurdie PJ, Holmes S (2013) Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8:e61217. https://doi.org/10.1371/journal.pone.0061217
Gentleman R, Carey V, Huber W, Hahne F (2019) Genefilter: methods for filtering genes from high-throughput experiments. R package version 1.66.0. https://bioconductor.org/packages/release/bioc/html/genefilter.html
Oksanen J, Blanchet FG, Kindt RLP, Minchin P, O’Hara RB, Simpson G, Solymos P, Stevens MHH (2015) Wagner H. Vegan: community ecology package. R package vegan. vers. 2.2–1
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253. https://doi.org/10.1111/j.1541-0420.2005.00440.x
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693. https://doi.org/10.1111/j.1461-0248.2006.00926.x
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12):121. https://doi.org/10.1186/s13059-014-0550-8
Jones JC, Fruciano C, Hildebrand F, Al Toufalilia H, Balfour NJ, Bork P, Engel P, Ratnieks FLW Hughes, W. O. (2018). Gut microbiota composition is associated with environmental landscape in honey bees. Ecol Evol 8(1): 441-451. https://doi.org/10.1002/ece3.3597
Kleinbaum DG, Klein M (2012) Kaplan-Meier survival curves and the log-rank test. In: Kleinbaum DG, Klein M (ed) Survival analysis. Statistics for Biology and Health. Springer, New York, NY, pp 55–96.
Tarek H, Hamiduzzaman MM, Morfin N, Guzman-Novoa E (2018) Sub-lethal doses of neonicotinoid and carbamate insecticides reduce the lifespan and alter the expression of immune health and detoxification related genes of honey bees (Apis mellifera). Genetics and Molecular Research 17 (2): gmr16039908. https://doi.org/10.4238/gmr16039908
Li Z, Li M, He J, Zhao X, Chaimanee V, Huang WF, Nie H, Zhao Y, Su S (2017) Differential physiological effects of neonicotinoid insecticides on honey bees: a comparison between Apis mellifera and Apis cerana. Pestic Biochem Physiol 140:1–8. https://doi.org/10.1016/j.pestbp.2017.06.010
Nelson CM, Ihle KE, Fondrk MK, Page RE, Amdam G (2007) The gene vitellogenin 444 has multiple coordinating effects on social organization. PLoS Biol 5(e62):445. https://doi.org/10.1371/journal.pbio.0050062
Moran NA, Hansen AK, Powell JE, Sabree ZL (2012) Distinctive gut microbiota of honey bees assessed using deep sampling from individual worker bees. PLoS ONE 7(4):e36393. https://doi.org/10.1371/journal.pone.0036393
Corby-Harris V, Maes P, Anderson KE (2014) The bacterial communities associated with honey bee (Apis mellifera) foragers. PLoS ONE 9:e95056. https://doi.org/10.1371/journal.pone.0095056
Castelli L, Branchiccela B, Garrido M, Invernizzi C, Porrini M, Romero H, Santos E, Zunino P, Antúnez K (2020) Impact of nutritional stress on honeybee gut microbiota, immunity, and Nosema ceranae infection. Microb Ecol 80(4):908–919. https://doi.org/10.1007/s00248-020-01538-1
Panjad P, Yongsawas R, Sinpoo C, Pakwan C, Subta P, Krongdang S, In-on A, Chomdej S, Chantawannakul P, Disayathanoowat T (2021) Impact of Nosema disease and American foulbrood on gut bacterial communities of honeybees Apis mellifera. Insects 12(6):525. https://doi.org/10.3390/insects12060525
Huang SK, Ye KT, Huang WF, Ying BH, Su X, Lin LH, Li JH, Chen YP, Li JL, Bao XL, Hu JZ (2018) Influence of feeding type and Nosema ceranae infection on the gut microbiota of Apis cerana workers. mSystems 3:e00177–18. https://doi.org/10.1128/mSystems.00177-18
Almasri H, Liberti J, Brunet JL, Belzunces LP (2022) Mild chronic exposure to pesticides alters physiological markers of honey bee health without perturbing the core gut microbiota. Sci Rep 12:4281. https://doi.org/10.1038/s41598-022-08009-2
Decourtye A, Devillers J, Cluzeau S, Charreton M, Pham-Delègue MH (2004) Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol Environ Saf 57:410–419. https://doi.org/10.1016/j.ecoenv.2003.08.001
Barker RJ, Lehner Y (1974) Acceptance and sustenance value of naturally occurring sugars fed to newly emerged adult workers of honey bees (Apis mellifera L.). J Exp Zoo 187:277–285
Niell S, Jesús F, Pérez N, Pérez C, Pareja L, Abbate S, Carrasco-Letelier L, Díaz S, Mendoza Y, Cesio V, Heinzen H (2017) Neonicotinoids transference from the field to the hive by honeybees: towards a pesticide residues biomonitor. Sci Total Environ 581:25–33. https://doi.org/10.1016/j.scitotenv.2017.01.011
Acknowledgements
The authors thank Enrique Nogueira and Pablo Juri from the Faculty of Veterinary Medicine, Universidad de la República, Montevideo, Uruguay, for the use of their experimental apiary and Jorge Harriet and Juan Campá from the Miguel Rubino Veterinary Laboratory-MGAP, for advices and providing N. ceranae–infected honey bees.
Funding
This study was funded by Agencia Nacional de Investigación e Innovación (ANII-FCE_1_2017_1_135810 and POS_NAC_2018_1_151557) and L’Oréal-UNESCO Award for Women in Science 2017.
Author information
Authors and Affiliations
Contributions
Conceptualization, P.Z. and K.A.; funding acquisition and project administration, K.A.; investigation and data analysis, S.B. and L.C.; writing—original draft, S.B. and K.A.; writing—review and editing, S.B., L.C., P.Z., and K.A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Balbuena, S., Castelli, L., Zunino, P. et al. Effect of Chronic Exposure to Sublethal Doses of Imidacloprid and Nosema ceranae on Immunity, Gut Microbiota, and Survival of Africanized Honey Bees. Microb Ecol 85, 1485–1497 (2023). https://doi.org/10.1007/s00248-022-02014-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-022-02014-8