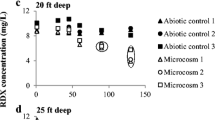
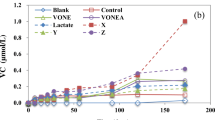
Abstract
In situ chemical oxidation with permanganate has become an accepted remedial treatment for groundwater contaminated with chlorinated solvents. This study focuses on the immediate and short-term effects of sodium permanganate (NaMnO4) on the indigenous subsurface microbial community composition in groundwater impacted by trichloroethylene (TCE). Planktonic and biofilm microbial communities were studied using groundwater grab samples and reticulated vitreous carbon passive samplers, respectively. Microbial community composition was analyzed by terminal restriction fragment length polymorphism and a high-density phylogenetic microarray (PhyloChip). Significant reductions in microbial diversity and biomass were shown during NaMnO4 exposure, followed by recovery within several weeks after the oxidant concentrations decreased to <1 mg/L. Bray–Curtis similarities and nonmetric multidimensional scaling showed that microbial community composition before and after NaMnO4 was similar, when taking into account the natural variation of the microbial communities. Also, 16S rRNA genes of two reductive dechlorinators (Desulfuromonas spp. and Sulfurospirillum spp.) and diverse taxa capable of cometabolic TCE oxidation were detected in similar quantities by PhyloChip across all monitoring wells, irrespective of NaMnO4 exposure and TCE concentrations. However, minimal biodegradation of TCE was observed in this study, based on oxidized conditions, concentration patterns of chlorinated and nonchlorinated hydrocarbons, geochemistry, and spatiotemporal distribution of TCE-degrading bacteria.





Similar content being viewed by others
References
Bakken LR, Olsen RA (1989) DNA-content of soil bacteria of different cell size. Soil Biol Biochem 6:789–793
Baldwin BR, Peacock AD, Park M, Ogles DM, Istok JD, McKinley JP, Resch CT, White DC (2008) Multilevel samplers as microcosms to assess microbial response to biostimulation. Ground Water 46:295–304
Bradley PM (2000) Microbial degradation of chloroethenes in groundwater systems. Hydrogeol J 8:104–111
Brodie EL, DeSantis TZ, Joyner DC, Baek SM, Larsen JT, Andersen GL, Hazen TC, Richardson PM, Herman DJ, Tokunaga TK, Wan JMM, Firestone MK (2006) Application of a high-density oligonucleotide microarray approach to study bacterial population dynamics during uranium reduction and reoxidation. Appl Environ Microbiol 72:6288–6298
Brodie EL, DeSantis TZ, Parker JPM, Zubietta IX, Piceno YM, Andersen GL (2007) Urban aerosols harbor diverse and dynamic bacterial populations. Proc Natl Acad Sci USA 104:299–304
Chang YP, Long PE, Geyer R, Peacock AD, Resch CT, Sublette K, Pfiffner S, Smithgall A, Anderson RT, Vrionis HA, Stephen JR, Dayvault R, Ortiz-Bernad I, Lovley DR, White DC (2005) Microbial incorporation of C-13-labeled acetate at the field scale: detection of microbes responsible for reduction of U(VI). Environ Sci Technol 39:9039–9048
Chen YM, Lin TF, Huang C, Lin JC (2008) Cometabolic degradation kinetics of TCE and phenol by Pseudomonas putida. Chemosphere 72:1671–1680
Christ JA, Ramsburg CA, Abriola LM, Pennell KD, Loeffler FW (2005) Coupling aggressive mass removal with microbial reductive dechlorination for remediation of DNAPL source zones: a review and assessment. Environ Health Perspect 113:465–477
Cichocka D, Siegert M, Imfeld G, Andert J, Beck K, Diekert G, Richnow HH, Nijenhuis I (2007) Factors controlling the carbon isotope fractionation of tetra- and trichloroethene during reductive dechlorination by Sulfurospirillum spp. and Desulfitobacterium sp. strain PCE-S. FEMS Microbiol Ecol 62:98–107
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. Primer-E, Plymouth, UK
Conrad ME, Brodie EL, Radtke CW, Corey W, Bill M, Delwiche ME, Lee MH, Swift DL, Colwell FS (2010) Field evidence for co-metabolism of trichloroethene stimulated by addition of electron donor to groundwater. Environ Sci Technol 44:4697–4704
Cordova-Kreylos AL, Cao YP, Green PG, Hwang HM, Kuivila KM, LaMontagne MG, Van De Werfhorst LC, Holden PA, Scow KM (2006) Diversity, composition, and geographical distribution of microbial communities in California salt marsh sediments. Appl Environ Microbiol 72:3357–3366
Crimi ML, Siegrist RL (2003) Geochemical effects on metals following permanganate oxidation of DNAPLs. Ground Water 41:458–469
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072
DeSantis TZ, Brodie EL, Moberg JP, Zubieta IX, Piceno YM, Andersen GL (2007) High-density universal 16S rRNA microarray analysis reveals broader diversity than typical clone library when sampling the environment. Microb Ecol 53:371–383
Drzyzga O, El Mamouni R, Agathos SN, Gottschal JC (2002) Dehalogenation of chlorinated ethenes and immobilization of nickel in anaerobic sediment columns under sulfidogenic conditions. Environ Sci Technol 36:2630–2635
Flynn TM, Sanford RA, Bethke CM (2008) Attached and suspended microbial communities in a pristine confined aquifer. Water Resour Res 44:1–7
Griebler C, Lueders T (2009) Microbial biodiversity in groundwater ecosystems. Freshw Biol 54:649–677
Griebler C, Mindl B, Slezak D, Geiger-Kaiser M (2002) Distribution patterns of attached and suspended bacteria in pristine and contaminated shallow aquifers studied with an in situ sediment exposure microcosm. Aquat Microb Ecol 28:117–129
Griffiths RI, Whiteley AS, O’Donnell AG, Bailey MJ (2000) Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl Environ Microbiol 66:5488–5491
Hendrickson ER, Payne JA, Young RM, Starr MG, Perry MP, Fahnestock S, Ellis DE, Ebersole RC (2002) Molecular analysis of Dehalococcoides 16S ribosomal DNA from chloroethene-contaminated sites throughout North America and Europe. Appl Environ Microbiol 68:485–495
Hohnstock-Ashe AM, Plummer SM, Yager RM, Baveye P, Madsen EL (2001) Further biogeochemical characterization of a trichloroethene-contaminated fractured dolomite aquifer: electron source and microbial communities involved in reductive dechlorination. Environ Sci Technol 35:4449–4456
Holm PE, Nielsen PH, Albrechtsen HJ, Christensen TH (1992) Importance of unattached bacteria and bacteria attached to sediment in determining potentials for degradation of xenobiotic organic contaminants in an aerobic aquifer. Appl Environ Microbiol 58:3020–3026
Honning J, Broholm MM, Bjerg PL (2007) Role of diffusion in chemical oxidation of PCE in a dual permeability system. Environ Sci Technol 41:8426–8432
Hrapovic L, Sleep BE, Major DJ, Hood ED (2005) Laboratory study of treatment of trichloroethene by chemical oxidation followed by bioremediation. Environ Sci Technol 39:2888–2897
Huling SG, Pivetz BE (2006) In-situ chemical oxidation. United States Environmental Protection Agency, Office of Research and Development, National Risk Management Research Laboratory, Cincinnati, OH
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei DG, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139:485–498
Kent AD, Smith DJ, Benson BJ, Triplett EW (2003) Web-based phylogenetic assignment tool for analysis of terminal restriction fragment length polymorphism profiles of microbial communities. Appl Environ Microbiol 69:6768–6776
Kikuchi T, Iwasaki K, Nishihara H, Takamura Y, Yagi O (2002) Quantitative and rapid detection of the trichloroethylene-degrading bacterium Methylocystis sp. M in groundwater by real-time PCR. Appl Microbiol Biotechnol 59:731–736
La Duc MT, Osman S, Vaishampayan P, Piceno Y, Andersen G, Spry JA, Venkateswaran K (2009) Comprehensive census of bacteria in clean rooms by using DNA microarray and cloning methods. Appl Environ Microbiol 75:6559–6567
LaMontagne MG, Holden PA (2003) Comparison of free-living and particle-associated bacterial communities in a coastal lagoon. Microbiol Ecol 46:228–237
LaMontagne MG, Leifer I, Bergmann S, Van De Werfhorst LC, Holden PA (2004) Bacterial diversity in marine hydrocarbon seep sediments. Environ Microbiol 6:799–808
Lehman RM, O’Connell SP, Banta A, Fredrickson JK, Rysenbach AL, Kieft TL, Colwell FS (2004) Microbiological comparison of core and groundwater samples collected from a fractured basalt aquifer with that of dialysis chambers incubated in situ. Geomicrobiol J 21:169–182
Liang YT, Van Nostrand JD, Wang J, Zhang X, Zhou JZ, Li GH (2009) Microarray-based functional gene analysis of soil microbial communities during ozonation and biodegradation of crude oil. Chemosphere 75:193–199
Macbeth TW, Cummings DE, Spring S, Petzke LM, Sorenson KS (2004) Molecular characterization of a dechlorinating community resulting from in situ biostimulation in a trichloroethene-contaminated deep, fractured basalt aquifer and comparison to a derivative laboratory culture. Appl Environ Microbiol 70:7329–7341
McGuire TM, McDade JM, Newell CJ (2006) Performance of DNAPL source depletion technologies at 59 chlorinated solvent-impacted sites. Ground Water Monit Remediat 26:73–84
Miller TR, Franklin MP, Halden RU (2007) Bacterial community analysis of shallow groundwater undergoing sequential anaerobic and aerobic chloroethene biotransformation. FEMS Microbiol Ecol 60:299–311
Moran MJ, Zogorski JS, Squillace PJ (2006) Chlorinated solvents in groundwater of the United States. Environ Sci Technol 41:74–81
Mundle K, Reynolds DA, West MR, Kueper BH (2007) Concentration rebound following in situ chemical oxidation in fractured clay. Ground Water 45:692–702
Nijenhuis I, Nikolausz M, Koth A, Felfolfi T, Weiss H, Drangmeister J, Grossmann J, Kastner M, Richnow HH (2007) Assessment of the natural attenuation of chlorinated ethenes in an anaerobic contaminated aquifer in the Bitterfeld/Wolfen area using stable isotope techniques, microcosm studies and molecular biomarkers. Chemosphere 67:300–311
Osborn AM, Moore ERB, Timmis KN (2000) An evaluation of terminal-restriction fragment length polymorphism (T-RFLP) analysis for the study of microbial community structure and dynamics. Environ Microbiol 2:39–50
Peacock AD, Chang YJ, Istok JD, Krumholz L, Geyer R, Kinsall B, Watson D, Sublette KL, White DC (2004) Utilization of microbial biofilms as monitors of bioremediation. Microb Ecol 47:284–292
Reardon CL, Cummings DE, Petzke LM, Kinsall BL, Watson DB, Peyton BM, Geesey GG (2004) Composition and diversity of microbial communities recovered from surrogate minerals incubated in an acidic uranium-contaminated aquifer. Appl Environ Microbiol 70:6037–6046
Richardson RE, James CA, Bhupathiraju VK, Alvarez-Cohen L (2002) Microbial activity in soils following steam treatment. Biodegradation 13:285–295
Rowe AR, Lazar BJ, Morris RM, Richardson RE (2008) Characterization of the community structure of a dechlorinating mixed culture and comparisons of gene expression in planktonic and biofloc-associated “Dehalococcoides” and Methanospirillum species. Appl Environ Microbiol 74:6709–6719
Sahl JW, Munakata-Marr J, Crimi ML, Siegrist RL (2007) Coupling permanganate oxidation with microbial dechlorination. Water Environ Res 79:5–12
Sercu B, Van De Werfhorst LC, Murray JLS, Holden PA (2011) Cultivation-independent analysis of bacteria in IDEXX Quanti-Tray/2000 fecal indicator assays. Appl Environ Microbiol 77:627–633
Sung Y, Ritalahti KM, Sanford RA, Urbance JW, Flynn SJ, Tiedje JM, Loeffler FE (2003) Characterization of two tetrachloroethene-reducing, acetate-oxidizing anaerobic bacteria and their description as Desulfuromonas michiganensis sp. nov. Appl Environ Microbiol 69:2964–2974
Suttinun O, Muller R, Luepromchai E (2009) Trichloroethylene cometabolic degradation by Rhodococcus sp. L4 induced with plant essential oils. Biodegradation 20:281–291
Thomson NR, Hood ED, Farquhar GJ (2007) Permanganate treatment of an emplaced DNAPL source. Ground Water Monit Remediat 27:74–85
Watts JR, Teel AL (2006) Treatment of contaminated soils and groundwater using ISCO. Pract Period Hazard Toxic Radioact Waste Manag 10:2–9
Yagi JM, Neuhauser EF, Ripp JA, Mauro DM, Madsen EL (2010) Subsurface ecosystem resilience: long-term attenuation of subsurface contaminants supports a dynamic microbial community. ISME J 4:131–143
Yergeau E, Schoondermark-Stolk SA, Brodie EL, Dejean S, DeSantis TZ, Goncalves O, Piceno YM, Andersen GL, Kowalchuk GA (2009) Environmental microarray analyses of Antarctic soil microbial communities. ISME J 3:340–351
Yeskis D, Zavala B (2002) Ground-water sampling guidelines for superfund and RCRA project managers. US EPA, Publication Number EPA 542-S-02-001
Acknowledgements
Microbiological field research and molecular analysis of field samples was performed by UCSB, and PhyloChip analysis was performed by the Lawrence Berkeley National Laboratory, through a research contract with ENVIRON International Corporation. Part of the work at Lawrence Berkeley National Laboratory was performed under the auspices of the US Department of Energy by the University of California, Lawrence Berkeley National Laboratory, under Contract DE-AC02-05CH11231. Groundwater sampling with geochemical analysis was performed by ENVIRON International Corporation through a private contract. The authors acknowledge the help of Annie Corbett and Kevin Huniu for field sampling and laboratory analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
Supplemental tables and figures are available, and a description of the optimization of DNA extraction methods for RVC samples (DOCX 619 kb)
Rights and permissions
About this article
Cite this article
Sercu, B., Jones, A.D.G., Wu, C.H. et al. The Influence of In Situ Chemical Oxidation on Microbial Community Composition in Groundwater Contaminated with Chlorinated Solvents. Microb Ecol 65, 39–49 (2013). https://doi.org/10.1007/s00248-012-0092-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-012-0092-0