Abstract
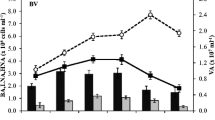
The influence of bacterial activity and diversity on bacterial growth efficiency was investigated in a flatland river. Eutrophic River Warnow drains predominantly agricultural land and is heavily loaded with nutrients, dissolved and particulate organic matter (DOM and POM), especially humic substances. Although the water column bacterial community consists of many inactive or damaged cells, bacterioplankton sustained a high bacterial secondary production of 0.2–14.5 μg C L−1 h−1 and a high DNA synthesis (thymidine uptake) of 6.1–15.5 μg C L−1 h−1. The direct and short-term measurement of bacterial respiration (by optodes) revealed high respiration rates especially in summer leading to directly estimated bacterial growth efficiencies (BGE) of 2–28%. These values are compared to calculations based only on bacterial production, which considerably overestimated BGEs. From all these data, River Warnow can be characterized as a strongly remineralizing system. River Warnow was dominated among others by Cytophaga/Flavobacteria and Actinobacteria which are typical for organic rich waters because of their ability to degrade high molecular weight compounds. However, community composition did not significantly affect BGE.





Similar content being viewed by others
References
Allgaier M, Grossart HP (2006) Seasonal dynamics and phylogenetic diversity of free-living and particle-associated bacterial communities in four lakes in northeastern Germany. Aquat Microb Ecol 45:115–128
Alonso-Saez L, Aristegui J, Pinhassi J, Gomez-Consarnau L, Gonzalez JM, Vaque D, Agusti S, Gasol JM (2007) Bacterial assemblage structure and carbon metabolism along a productivity gradient in the NE Atlantic Ocean. Aquat Microb Ecol 46:43–53
Alonso-Saez L, Vazquez-Dominguez E, Cardelus C, Pinhassi J, Sala MM, Lekunberri I, Balague V, Vila-Costa M, Unrein F, Massana R, Simo R, Gasol JM (2008) Factors controlling the year-round variability in carbon flux through bacteria in a coastal marine system. Ecosystems 11:397–409
Amon RMW, Benner R (1996) Bacterial utilization of different size classes of dissolved organic matter. Limnol Oceanogr 41:41–51
Anesio AM, Graneli W, Aiken GR, Kieber DJ, Mopper K (2005) Effect of humic substance photodegradation on bacterial growth and respiration in lake water. Appl Environ Microbiol 71:6267–6275
Azam F, Fenchel T, Field JG, Gray JS, Meyer Reil LA, Thingstad F (1983) The ecological role of water–column microbes in the sea. Mar Ecol Prog Ser 10:257–263
Bell RT (1993) Estimating production of heterotrophic bacterioplankton via incorporation of tritiated thymidine. Handb Aquat Microb Meth 495–503
Biddanda BA, Cotner JB (2003) Enhancement of dissolved organic matter bioavailability by sunlight and its role in the carbon cycle of Lakes Superior and Michigan. J Great Lakes Res 29:228–241
Biddanda BA, Ogdahl M, Cotner J (2001) Dominance of bacterial metabolism in oligotrophic relative to eutrophic waters. Limnol Oceanogr 46:730–739
Carlson CA, Ducklow HW (1996) Growth of bacterioplankton and consumption of dissolved organic carbon in the Sargasso Sea. Aquat Microb Ecol 10:69–85
Choi JW, Sherr BF, Sherr EB (1999) Dead or alive? A large fraction of ETS-inactive marine bacterioplankton cells, as assessed by reduction of CTC, can become ETS-active with incubation and substrate addition. Aquat Microb Ecol 18:105–115
Cleven EJ, Weisse T (2001) Seasonal succession and taxon-specific bacterial grazing rates of heterotrophic nanoflagellates in Lake Constance. Aquat Microb Ecol 23:147–161
Cottrell MT, Kirchman DL (2000) Natural assemblages of marine proteobacteria and members of the cytophaga-flavobacter cluster consuming low- and high-molecular weight-dissolved organic matter. Appl Environ Microbiol 66:1692–1697
Cottrell MT, Kirchman DL (2003) Contribution of major bacterial groups to bacterial biomass production (thymidine and leucine incorporation) in the Delaware estuary. Limnol Oceanogr 48:168–178
del Giorgio PA, Cole JJ (1998) Bacterial growth efficiency in natural aquatic systems. Annu Rev Ecol Evol Syst 29:503–541
del Giorgio PA, Scarborough G (1995) Increase in the proportion of metabolically active bacteria along gradients of enrichment in freshwater and marine plankton—implications for estimates of bacterial growth and production rates. J Plankton Res 17:1905–1924
Eiler A, Langenheder S, Bertilsson S, Tranvik LJ (2003) Heterotrophic bacterial growth efficiency and community structure at different natural organic carbon concentrations. Appl Environ Microbiol 69:3701–3709
Freese HM, Karsten U, Schumann R (2006) Bacterial abundance, activity, and viability in the Eutrophic River Warnow, Northeast Germany. Microb Ecol 51:117–127
Freese HM, Görs S, Karsten U, Schumann R (2007) Dissolved inorganic nutrients and organic substrates in the River Warnow (North-Eastern Germany)—utilisation by bacterioplankton. Limnologica 37:264–277
Fuhrman JA, Azam F (1980) Bacterioplankton secondary production estimates for coastal waters of British Columbia, Antarctica, and California. Appl Environ Microbiol 39:1085–1095
Gasol JM, Aristegui J (2007) Cytometric evidence reconciling the toxicity and usefulness of CTC as a marker of bacterial activity. Aquat Microb Ecol 46:71–83
Gasol JM, del Giorgio PA, Massana R, Duarte CM (1995) Active versus inactive bacteria: size-dependence in a coastal marine plankton community. Mar Ecol Prog Ser 128:91–97
Gasol JM, Pinhassi J, Alonso-Saez L, Ducklow H, Herndl GJ, Koblizek M, Labrenz M, Luo Y, Moran XAG, Reinthaler T, Simon M (2008) Towards a better understanding of microbial carbon flux in the sea. Aquat Microb Ecol 53:21–38
Geller A (1983) Degradability of dissolved organic lake water compounds in cultures of natural bacterial communities. Arch Hydrobiol 99:60–79
Glöckner FO, Zaichikov E, Belkova N, Denissova L, Pernthaler J, Pernthaler A, Amann R (2000) Comparative 16S rRNA analysis of lake bacterioplankton reveals globally distributed phylogenetic clusters including an abundant group of actinobacteria. Appl Environ Microbiol 66:5053–5065
Görs S, Rentsch D, Schiewer U, Karsten U, Schumann R (2007) Dissolved organic matter along the eutrophication gradient of the Darss-Zingst Bodden Chain. Southern Baltic Sea: I. Chemical characterisation and composition. Mar Chem 104:125–142
Hall EK, Cotner JB (2007) Interactive effect of temperature and resources on carbon cycling by freshwater bacterioplankton communities. Aquat Microb Ecol 49:35–45
Haukka K, Heikkinen E, Kairesalo T, Karjalainen H, Sivonen K (2005) Effect of humic material on the bacterioplankton community composition in boreal lakes and mesocosms. Environ Microbiol 7:620–630
Jorgensen NOG (1987) Free amino acids in lakes—concentrations and assimilation rates in relation to phytoplankton and bacterial production. Limnol Oceanogr 32:97–111
Kirchman DL (1990) Limitation of bacterial growth by dissolved organic matter in the Sub-Arctic Pacific. Mar Ecol Prog Ser 62:47–54
Kirchman DL (2002) The ecology of Cytophaga–Flavobacteria in aquatic environments. FEMS Microbiol Ecol 39:91–100
Kirchman DL, K'nees E, Hodson R (1985) Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl Environ Microbiol 49:599–607
Kirchman DL, Meon B, Ducklow HW, Carlson CA, Hansell DA, Steward GF (2001) Glucose fluxes and concentrations of dissolved combined neutral sugars (polysaccharides) in the Ross Sea and Polar Front Zone, Antarctica. Deep Sea Res II 48:4179–4197
Moran MA, Hodson RE (1990) Bacterial production on humic and nonhumic components of dissolved organic carbon. Limnol Oceanogr 35:1744–1756
Münster U (1993) Concentrations and fluxes of organic carbon substrates in the aquatic environment. Anton Leeuw Int J G 63:243–274
Pernthaler A, Permthaler J, Amann R (2002) Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl Environ Microbiol 68:3094–3101
Pinhassi J, Berman T (2003) Differential growth response of colony-forming α- and γ-proteobacteria in dilution culture and nutrient addition experiments from Lake Kinneret (Israel), the Eastern Mediterranean Sea, and the Gulf of Eilat. Appl Environ Microbiol 69:199–211
Pomeroy LR, Wiebe WJ (2001) Temperature and substrates as interactive limiting factors for marine heterotrophic bacteria. Aquat Microb Ecol 23:187–204
Pomeroy LR, Wiebe WJ, Deibel D, Thompson RJ, Rowe GT, Pakulski JD (1991) Bacterial responses to temperature and substrate concentration during the newfoundland spring bloom. Mar Ecol Prog Ser 75:143–159
Riemann L, Steward GF, Azam F (2000) Dynamics of bacterial community composition and activity during a mesocosm diatom bloom. Appl Environ Microbiol 66:578–587
Rodrigues RMNV, Williams PJl (2001) Heterotrophic bacterial utilization of nitrogenous and nonnitrogenous substrates, determined from ammonia and oxygen fluxes. Limnol Oceanogr 46:1675–1683
Schumann R, Rieling T, Görs S, Hammer A, Selig U, Schiewer U (2003) Viability of bacteria from different aquatic habitats. I. Environmental conditions and productivity. Aquat Microb Ecol 32:121–135
Schwaerter S, Soendergaard M, Riemann B, Moeller Jensen L (1988) Respiration in eutrophic lakes: the contribution of bacterioplankton and bacterial growth yield. J Plankton Res 10:515–531
Sekar R, Pernthaler A, Pernthaler J, Warnecke F, Posch T, Amann R (2003) An improved protocol for quantification of freshwater actinobacteria by fluorescence in situ hybridization. Appl Environ Microbiol 69:2928–2935
Shiah F-K, Ducklow HW (1994) Temperature and substrate regulation of bacterial abundance, production and specific growth rate in Chesapeake Bay, USA. Mar Ecol Prog Ser 103:297–308
Simon M, Azam F (1989) Protein content and protein synthesis rates of planktonic marine bacteria. Mar Ecol Prog Ser 51:201–213
Smits JD, Riemann B (1988) Calculation of cell production from [3H]-thymidine incorporation with freshwater bacteria. Appl Environ Microbiol 54:2213–2219
Sugimura Y, Suzuki Y (1988) A high-temperature catalytic-oxidation method for the determination of non-volatile dissolved organic carbon in seawater by direct injection of a liquid sample. Mar Chem 24:105–131
Thurman EM (1985) Organic geochemistry of natural waters. Kluwer Academic Publishers, Boston
Tranvik LJ, Höfle MG (1987) Bacterial growth in mixed cultures on dissolved organic carbon from humic and clear waters. Appl Environ Microbiol 53:482–488
Vadstein O, Harkjerr BO, Jensen A, Olsen Y, Reinertsen H (1989) Cycling of organic carbon in the photic zone of a eutrophic lake with special reference to the heterotrophic bacteria. Limnol Oceanogr 34:840–855
Warkentin M, Freese HM, Karsten U, Schumann R (2007) New and fast method to quantify respiration rates of bacterial and plankton communities in freshwater ecosystems by using optical oxygen sensor spots. Appl Environ Microbiol 73:6722–6729
White PA, Kalff J, Rasmussen JB, Gasol JM (1991) The effect of temperature and algal biomass on bacterial production and specific growth rate in freshwater and marine habitats. Microb Ecol 21:99–118
Wiebe WJ, Sheldon WM Jr, Pomeroy LR (1992) Bacterial growth in the cold: evidence for an enhanced substrate requirement. Appl Environ Microbiol 58:359–364
Wiebe WJ, Sheldon WM, Pomeroy LR (1993) Evidence for an enhanced substrate requirement by marine mesophilic bacterial isolates at minimal growth temperatures. Microb Ecol 25:151–159
Zwart G, Crump BC, Agterveld MPKV, Hagen F, Han SK (2002) Typical freshwater bacteria: an analysis of available 16S rRNA gene sequences from plankton of lakes and rivers. Aquat Microb Ecol 28:141–155
Acknowledgements
We thank H.P. Grossart (IGB Berlin) for his help with glucose analytics. This work was supported by a grant from the Ministry of Education, Science and Culture Mecklenburg-Vorpommern, Germany, to H.M. Freese. We are grateful for the interesting discussion with and valuable suggestions made by all reviewers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Warkentin, M., Freese, H.M. & Schumann, R. Bacterial Activity and Bacterioplankton Diversity in the Eutrophic River Warnow—Direct Measurement of Bacterial Growth Efficiency and Its Effect on Carbon Utilization. Microb Ecol 61, 190–200 (2011). https://doi.org/10.1007/s00248-010-9729-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-010-9729-z