Abstract
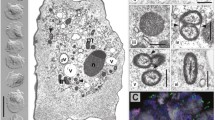
The anaerobic free-living ciliate, Trimyema compressum, is known to harbor both methanogenic archaeal and bacterial symbionts in the cytoplasm. To clarify their phylogenetic belongings, a full-cycle rRNA approach was applied to this symbiosis. Phylogenetic analysis showed that the methanogenic symbiont was related to Methanobrevibacter arboriphilicus, which was distantly related to symbionts found in other Trimyema species. This result suggested that Trimyema species do not require very specific methanogenic symbionts, and symbiont replacement could have occurred in the history of Trimyema species. On the other hand, the bacterial symbiont was located near the lineage of the family Syntrophomonadaceae in the phylum Firmicutes. The sequence similarity between the bacterial symbiont and the nearest species was 85%, indicating that bacterial symbionts may be specific to the Trimyema species. The elimination of bacterial symbionts from the ciliate cell by antibiotic treatment resulted in considerably decreased host growth. However, it was not restored by stigmasterol addition (<2 μg ml−1), which was different from the previous report that showed that the symbiont-free strain required exogenous sterols for growth. In addition, the decline of host growth was not accompanied by host metabolism shift toward the formation of more reduced products, which suggested that the contribution of bacterial symbionts to the host ciliate was not a dispose of excessive reducing equivalent arising from the host’s fermentative metabolism as methanogenic symbionts do. This study showed that bacterial symbionts make a significant contribution to the host ciliate by an unknown function and suggested that interactions between bacterial symbionts and T. compressum are more complicated than hitherto proposed.




Similar content being viewed by others
References
Burggraf, S, Olsen, GJ, Stetter, KO, Woese, CR (1992) A phylogenetic analysis of Aquifex pyrophilus. Syst Appl Microbiol 15: 352–356
Eder, W, Ludwig, W, Huber, R (1999) Novel 16S rRNA gene sequences retrieved from highly saline brine sediments of Kebrit Deep, Red Sea. Arch Microbiol 172: 213–218
Embley, TM, Finlay, BJ (1994) The use of small subunit rRNA sequences to unravel the relationships between anaerobic ciliates and their methanogen endosymbionts. Microbiology 140: 225–235
Embley, TM, Finlay, BJ (1993) Systematic and morphological diversity of endosymbiotic methanogens in anaerobic ciliates. Antonie Van Leeuwenhoek 64: 261–271
Embley, TM, Finlay, BJ, Thomas, RH, Dyal, PL (1992) The use of rRNA sequences and fluorescent probes to investigate the phylogenetic positions of the anaerobic ciliate Metopus palaeformis and its archaeobacterial endosymbiont. J Gen Microbiol 138: 1479–1487
Fenchel, T, Finlay, BJ (1991) Endosymbiotic methanogenic bacteria in anaerobic ciliates: significance for the growth efficiency of the host. J Protozool 38: 18–22
Finlay, BJ, Embley, TM, Fenchel, T (1993) A new polymorphic methanogen, closely related to Methanocorpusculum parvum, living in stable symbiosis within the anaerobic ciliate Trimyema sp. J Gen Microbiol 139: 371–378
Goosen, NK, Van der Drift, C, Stumm, CK, Vogels, GD (1990) End products of metabolism in the anaerobic ciliate Trimyema compressum. FEMS Microbiol Lett 69: 171–176
Goosen, NK, Wagener, S, Stumm, CK (1990) A comparison of two strains of the anaerobic ciliate Trimyema compressum. Arch Microbiol 153: 187–192
Hackstein, JH, Vogels, GD (1997) Endosymbiotic interactions in anaerobic protozoa. Antonie Van Leeuwenhoek 71: 151–158
Hackstein, JH, van Hoek, AH, Leunissen, JA, Huynen, M (2002) Anaerobic ciliates and their methanogenic endosymbionts. In: Seckbach, J (Ed.) Symbiosis: Mechanisms and Model Systems, Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 451–464
Hengstmann, U, Chin, KJ, Janssen, PH, Liesack, W (1999) Comparative phylogenetic assignment of environmental sequences of genes encoding 16S rRNA and numerically abundant culturable bacteria from an anoxic rice paddy soil. Appl Environ Microbiol 65: 5050–5058
Holler, S, Pfennig, N (1991) Fermentation products of the anaerobic ciliate Trimyema compressum in monoxenic cultures. Arch Microbiol 156: 327–334
Ishii, S, Kosaka, T, Hori, K, Hotta, Y, Watanabe, K (2005) Coaggregation facilitates interspecies hydrogen transfer between Pelotomaculum thermopropionicum and Methanothermobacter thermautotrophicus. Appl Environ Microbiol 71: 7838–7845
Lane, D (1991) 16S/23S rRNA sequencing. In: Stackebrandt, E, Goodfellow M (Eds.) Nucleic Acid Techniques in Bacterial Systematics, Wiley, Chichester, England, pp 115–175
Lin, C, Miller, TL (1998) Phylogenetic analysis of Methanobrevibacter isolated from feces of humans and other animals. Arch Microbiol 169: 397–403
López-García, P, Philippe, H, Gail, F, Moreira, D (2002) Autochthonous eukaryotic diversity in hydrothermal sediment and experimental microcolonizers at the Mid-Atlantic Ridge. Proc Natl Acad Sci USA 100: 697–702
Maidak, BJ, Cole, JR, Lilburn, TG, Parker, Jr, CT, Saxman, PR, Farris, RJ, Garrity, GM, Olsen, GJ, Schmidt, TM, Tiedje, JM (2001) The RDP-II (Ribosomal Database Project). Nucleic Acids Res 29: 173–174
Mori, K, Yamamoto, H, Kamagata, Y, Hatsu, M, Takamizawa, K (2000) Methanocalculus pumilus sp. nov., a heavy-metal-tolerant methanogen isolated from a waste-disposal site. Int J Syst Evol Microbiol 50: 1723–1729
Müller, M (1988) Energy metabolism of protozoa without mitochondria. Annu Rev Microbiol 42: 465–488
Saitou, N, Nei, M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425
Schink, B (1997) Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev 61: 262–280
Sekiguchi, Y, Kamagata, Y, Nakamura, K, Ohashi, A, Harada, H (1999) Fluorescence in situ hybridization using 16S rRNA-targeted oligonucleotides reveals localization of methanogens and selected uncultured bacteria in mesophilic and thermophilic sludge granules. Appl Environ Microbiol 65: 1280–1288
van Bruggen, JJA, Zwart, KB, Van Assema, RM, Stumm, CK, Vogels, GD (1984) Methanobacterium formicicum, an endosymbiont of the anaerobic ciliate Metopus striatus McMurrich. Arch Microbiol 139: 1–7
van Bruggen, JJA, Zwart, KB, Hermans, JGF, Stumm, CK, Vogels, GD (1986) Isolation and characterization of Methanoplanus endosymbiosus sp. nov., an endosymbiont of the marine sapropelic ciliate Metopus contortus Quennerstedt. Arch Microbiol 144: 367–374
van Bruggen, JJA, Stumm, CK, Vogels, GD (1983) Symbiosis of methanogenic bacteria and sapropelic protozoa. Arch Microbiol 136: 89–95
van Hoek, AH, van Alen, TA, Sprakel, VS, Leunissen, JA, Brigge, T, Vogels, GD, Hackstein, JH (2000) Multiple acquisition of methanogenic archaeal symbionts by anaerobic ciliates. Mol Biol Evol 17: 251–258
Wagener, S, Bardele, CF, Pfennig, N (1990) Functional integration of Methanobacterium formicicum into the anaerobic ciliate Trimyema compressum. Arch Microbiol 153: 496–501
Wagener, S, Pfennig, N (1987) Monoxenic culture of the anaerobic ciliate Trimyema compressum Lackey. Arch Microbiol 149: 4–11
Yamada, K, Kamagata, Y, Nakamura, K (1997) The effect of endosymbiotic methanogens on the growth and metabolic profile of the anaerobic free-living ciliate Trimyema compressum. FEMS Microbiol Lett 149: 129–132
Yamada, K, Kamagata, Y, Nakamura, K, Inamori, Y, Nakamura, I (1994) Selectivity of food bacteria for the growth of anaerobic ciliate Trimyema compressum. Arch Microbiol 161: 229–233
Acknowledgments
We thank Miho Enoki (Marine Biotechnology Institute) for maintaining T. compressum cultures. This work was supported by the New Energy and Industrial Technology Development Organization (NEDO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shinzato, N., Watanabe, I., Meng, XY. et al. Phylogenetic Analysis and Fluorescence In Situ Hybridization Detection of Archaeal and Bacterial Endosymbionts in the Anaerobic Ciliate Trimyema Compressum . Microb Ecol 54, 627–636 (2007). https://doi.org/10.1007/s00248-007-9218-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-007-9218-1