Abstract
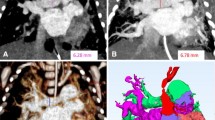
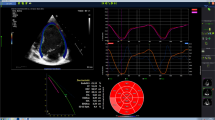
High-resolution, isotropic, 3-dimensional (D) data from pediatric cardiovascular computed tomography (CT) offer great potential for the accurate quantitative evaluation of pediatric cardiovascular and pulmonary vascular diseases. Recent pilot studies using pediatric 3-D cardiovascular CT have shown promising results in assessing cardiac function in conditions such as tetralogy of Fallot, cardiac defects with a hypoplastic ventricle, Ebstein anomaly, and in quantifying myocardial mass. In addition, the quantitative assessment of pulmonary vascularity is useful for evaluating differential right-to-left pulmonary vascular volume ratio, the effectiveness of pulmonary angioplasty, and predicting pulmonary hypertension. These initial experiences could broaden the role of pediatric cardiovascular CT in clinical practice. Furthermore, the current barriers to its widespread use, pertinent solutions to these problems, and new applications are discussed. In this review, the 3-D quantitative evaluations of cardiac function and pulmonary vascularity using high-resolution pediatric cardiovascular CT data are illustrated.









Similar content being viewed by others
Data availability
Data will be available upon reasonable request.
References
Goo HW, Park IS, Ko JK et al (2003) CT of congenital heart disease: normal anatomy and typical pathologic conditions. Radiographics 23:S147–S165
Goo HW, Siripornpitak S, Chen S et al (2021) Pediatric cardiothoracic computed tomography guideline provided by the Asian Society of Cardiovascular Imaging (ASCI) Congenital Heart Disease Study Group: Part 2. Contemporary clinical applications. Korean J Radiol 22:1397–1415
Hofmann LK, Becker CR, Flohr T, Schoepf UJ (2003) Multidetector-row CT of the heart. Semin Roentgenol 38:135–145
Goo HW, Yang DH (2010) Coronary artery visibility in free-breathing young children with congenital heart disease on cardiac 64-slice CT: dual-source ECG-triggered sequential scan vs. single-source non-ECG-synchronized spiral scan. Pediatr Radiol 40:1670–1680
Pache G, Grohmann J, Bulla S et al (2011) Prospective electrocardiography-triggered CT angiography of the great thoracic vessels in infants and toddlers with congenital heart disease: feasibility and image quality. Eur J Radiol 80:e440–e445
Nakagawa M, Ozawa Y, Nomura N et al (2016) Utility of dual source CT with ECG-triggered high-pitch spiral acquisition (Flash Spiral Cardio mode) to evaluate morphological features of ventricles in children with complex congenital heart defects. Jpn J Radiol 34:284–291
Hui PKT, Goo HW, Du J et al (2017) Asian consortium on radiation dose of pediatric cardiac CT (ASCI-REDCARD). Pediatr Radiol 47:899–910
Rigsby CK, McKenney SE, Hill KD et al (2018) Radiation dose management for pediatric cardiac computed tomography: a report from the Image Gently ‘Have-A-Heart’ campaign. Pediatr Radiol 48:5–20
Hong SW, Goo HW, Maeda E et al (2019) User-friendly, vendor-specific guideline for pediatric cardiothoracic computed tomography provided by the Asian Society of Cardiovascular Imaging (ASCI) Congenital Heart Disease Study Group: Part 1. Imaging techniques. Korean J Radiol 20:190–204
Francone M, Gimelli A, Budde RPJ et al (2022) Radiation safety for cardiovascular computed tomography imaging in paediatric cardiology: a joint expert consensus document of the EACVI, ESCR, AEPC, and ESPR. Eur Heart J Cardiovasc Imaging 23:e279–e289
Horst KK, Yu L, McCollough CH et al (2023) Potential benefits of photon counting detector computed tomography in pediatric imaging. Br J Radiol 96:20230189
Juergens KU, Grude M, Maintz D et al (2004) Multi-detector row CT of left ventricular function with dedicated analysis software versus MR imaging: initial experience. Radiology 230:403–410
Goo HW, Park SH (2015) Semiautomatic three-dimensional CT ventricular volumetry in patients with congenital heart disease: agreement between two methods with different user interaction. Int J Cardiovasc Imaging 31(Suppl 2):223–232
Goo HW (2017) Myocardial delayed enhancement CT: initial experience in children and young adults. Pediatr Radiol 47:1452–1462
Takx RA, Moscariello A, Schoepf UJ et al (2012) Quantification of left and right ventricular function and myocardial mass: comparison of low-radiation dose 2nd generation dual-source CT and cardiac MRI. Eur J Radiol 81:e598–e604
Kim JY, Suh YJ, Han K et al (2020) Cardiac CT for measurement of right ventricular volume and function in comparison with cardiac MRI: a meta-analysis. Korean J Radiol 21:450–461
Sugeng L, Mor-Avi V, Weinert L et al (2010) Multimodality comparison of quantitative volumetric analysis of the right ventricle. JACC Cardiovasc Imaging 3:10–18
Retson TA, Masutani EM, Golden D, Hsiao A (2020) Clinical performance and role of expert supervision of deep learning for cardiac ventricular volumetry: a validation study. Radiol Artif Intell 2:e190064
Koch K, Oellig F, Oberholzer K et al (2005) Assessment of right ventricular function by 16-detector-row CT: comparison with magnetic resonance imaging. Eur Radiol 15:312–318
Goo HW, Park SH (2018) Computed tomography-based ventricular volumes and morphometric parameters for deciding the treatment strategy in children with a hypoplastic left ventricle: preliminary results. Korean J Radiol 19:1042–1052
Goo HW (2019) Semiautomatic three-dimensional threshold-based cardiac computed tomography ventricular volumetry in repaired tetralogy of Fallot: comparison with cardiac magnetic resonance imaging. Korean J Radiol 20:102–113
Stout KK, Daniels CJ, Aboulhosn JA et al (2019) 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 139:e698–e800
Goo HW (2019) Changes in right ventricular volume, volume load, and function measured with cardiac computed tomography over the entire time course of tetralogy of Fallot. Korean J Radiol 20:956–966
Grothoff M, Hoffmann J, Lehmkuhl L et al (2011) Time course of right ventricular functional parameters after surgical correction of tetralogy of Fallot determined by cardiac magnetic resonance. Clin Res Cardiol 100:343–350
Śpiewak M, Małek ŁA, Petryka J et al (2013) Stable right ventricular size and function during short-term follow-up in patients with pulmonary regurgitation after tetralogy of Fallot repair. Clin Radiol 68:1206–1211
Buddhe S, Shah A, Lai WW (2015) Progression of right ventricular dilation in repaired tetralogy of Fallot. J Magn Reson Imaging 41:730–737
Rutz T, Ghandour F, Meierhofer C et al (2017) Evolution of right ventricular size over time after tetralogy of Fallot repair: a longitudinal cardiac magnetic resonance study. Eur Heart J Cardiovasc Imaging 18:364–370
Hoelscher M, Bonassin F, Oxenius A et al (2020) Right ventricular dilatation in patients with pulmonary regurgitation after repair of tetralogy of Fallot: how fast does it progress? Ann Pediatr Cardiol 13:294–300
Goo HW, Park SH (2023) Identification of rapid progression of right ventricular functional measures using three-dimensional cardiac computed tomography after total surgical correction of tetralogy of Fallot. Eur J Radiol 164:110856
Heng EL, Gatzoulis MA, Uebing A et al (2017) Immediate and midterm cardiac remodeling after surgical pulmonary valve replacement in adults with repaired tetralogy of Fallot: a prospective cardiovascular magnetic resonance and clinical study. Circulation 136:1703–1713
Lee C, Choi ES, Lee CH (2020) Long-term outcomes of pulmonary valve replacement in patients with repaired tetralogy of Fallot. Eur J Cardiothorac Surg 58:246–252
Gursu HA, Varan B, Sade E et al (2016) Analysis of right ventricle function with strain imaging before and after pulmonary valve replacement. Cardiol J 23:195–201
Hallbergson A, Gauvreau K, Powell AJ, Geva T (2015) Right ventricular remodeling after pulmonary valve replacement: early gains, late losses. Ann Thorac Surg 99:660–666
Tuo G, Khambadkone S, Tann O et al (2013) Obstructive left heart disease in neonates with a “borderline” left ventricle: diagnostic challenges to choosing the best outcome. Pediatr Cardiol 34:1567–1576
Kaplinski M, Cohen MS (2015) Characterising adequacy or inadequacy of the borderline left ventricle: what tools can we use? Cardiol Young 25:1482–1488
van Son JA, Phoon CK, Silverman NH, Haas GS (1997) Predicting feasibility of biventricular repair of right-dominant unbalanced atrioventricular canal. Ann Thorac Surg 63:1657–1663
Grosse-Wortmann L, Yun TJ, Al-Radi O et al (2008) Borderline hypoplasia of the left ventricle in neonates: insights for decision-making from functional assessment with magnetic resonance imaging. J Thorac Cardiovasc Surg 136:1429–1436
Goo HW (2017) Serial changes in anatomy and ventricular function on dual-source cardiac computed tomography after the Norwood procedure for hypoplastic left heart syndrome. Pediatr Radiol 47:1776–1786
Yoshimura N, Yamaguchi M (2009) Surgical strategy for pulmonary atresia with intact ventricular septum: initial management and definitive surgery. Gen Thorac Cardiovasc Surg 57:338–346
De Oliveira NC, Sittiwangkul R, McCrindle BW et al (2005) Biventricular repair in children with atrioventricular septal defects and a small right ventricle: anatomic and surgical considerations. J Thorac Cardiovasc Surg 130:250–257
Cinteză EE, Nicolescu AM, Iancu MA et al (2022) Isolated hypoplastic right ventricle - a challenge in medical practice. Rom J Morphol Embryol 63:49–53
Goo HW (2020) Quantification of initial right ventricular dimensions by computed tomography in infants with congenital heart disease and a hypoplastic right ventricle. Korean J Radiol 21:203–209
Carpentier A, Chauvaud S, Macé L et al (1988) A new reconstructive operation for Ebstein’s anomaly of the tricuspid valve. J Thorac Cardiovasc Surg 96:92–101
Celermajer DS, Bull C, Till JA et al (1994) Ebstein’s anomaly: presentation and outcome from fetus to adult. J Am Coll Cardiol 23:170–176
Hösch O, Sohns JM, Nguyen TT et al (2014) The total right/left-volume index: a new and simplifed cardiac magnetic resonance measure to evaluate the severity of Ebstein anomaly of the tricuspid valve: a comparison with heart failure markers from various modalities. Circ Cardiovasc Imaging 7:601–609
Rydman R, Shiina Y, Diller GP et al (2018) Major adverse events and atrial tachycardia in Ebstein’s anomaly predicted by cardiovascular magnetic resonance. Heart 104:37–44
Goo HW (2019) Volumetric severity assessment of Ebstein anomaly using three-dimensional cardiac CT: a feasibility study. Cardiovasc Imaging Asia 3:61–67
Goo HW, Park SH (2024) Complimentary cardiac computed tomography ventricular volumetry-derived metrics of severity in patients with Ebstein anomaly: comparison with echocardiography-based severity indices. Pediatr Cardiol 45:24–31
Malayeri AA, Johnson WC, Macedo R et al (2008) Cardiac cine MRI: quantification of the relationship between fast gradient echo and steady-state free precession for determination of myocardial mass and volumes. J Magn Reson Imaging 28:60–66
Gregor Z, Kiss AR, Szabó LE et al (2021) Sex- and age- specific normal values of left ventricular functional and myocardial mass parameters using threshold-based trabeculae quantification. PLoS ONE 16:e0258362
Freling HG, van Wijk K, Jaspers K et al (2013) Impact of right ventricular endocardial trabeculae on volumes and function assessed by CMR in patients with tetralogy of Fallot. Int J Cardiovasc Imaging 29:625–631
Goo HW, Park SH (2020) Pattern analysis of left ventricular remodeling using cardiac computed tomography in children with congenital heart disease: preliminary results. Korean J Radiol 21:717–725
Goo HW (2021) Right ventricular mass quantification using cardiac CT and a semiautomatic three-dimensional hybrid segmentation approach: a pilot study. Korean J Radiol 22:901–911
Mergen V, Sartoretti T, Klotz E et al (2022) Extracellular volume quantification with cardiac late enhancement scanning using dual-source photon-counting detector CT. Invest Radiol 57:406–411
Aquino GJ, O’Doherty J, Schoepf UJ et al (2023) Myocardial characterization with extracellular volume mapping with a first-generation photon-counting detector CT with MRI reference. Radiology 307:e222030
Pontone G, Muscogiuri G, Andreini D et al (2016) The new frontier of cardiac computed tomography angiography: fractional flow reserve and stress myocardial perfusion. Curr Treat Options Cardiovasc Med 18:74
Adjedj J, Hyafil F, Halna du Fretay X et al (2021) Physiological evaluation of anomalous aortic origin of a coronary artery using computed tomography-derived fractional flow reserve. J Am Heart Assoc 10:e018593
Goo HW (2021) Quantitative evaluation of coronary artery visibility on CT angiography in Kawasaki disease: young vs. old children. Int J Cardiovasc Imaging 37:1085–1092
Scannell CM, Hasaneen H, Greil G et al (2021) Automated quantitative stress perfusion cardiac magnetic resonance in pediatric patients. Front Pediatr 9:699497
Tumkosit M, Yingyong N, Mahayosnond A et al (2012) Accuracy of chest radiography for evaluating significantly abnormal pulmonary vascularity in children with congenital heart disease. Int J Cardiovasc Imaging 28(Suppl 1):69–75
Muster AJ, Paul MH, Van Grondelle A, Conway JJ (1976) Asymmetric distribution of the pulmonary blood flow between the right and left lungs in d-transposition of the great arteries. Am J Cardiol 38:352–361
Sridharan S, Derrick G, Deanfield J, Taylor AM (2006) Assessment of differential branch pulmonary blood flow: a comparative study of phase contrast magnetic resonance imaging and radionuclide lung perfusion imaging. Heart 92:963–968
Shin SM, Kim HK, Crotty EJ et al (2020) CT angiography findings of pulmonary arteriovenous malformations in children and young adults with hereditary hemorrhagic telangiectasia. AJR Am J Roentgenol 214:1369–1376
Goo HW (2010) Initial experience of dual-energy lung perfusion CT using a dual-source CT system in children. Pediatr Radiol 40:1536–1544
Goo HW, Park SH (2017) Pulmonary vascular volume ratio measured by cardiac computed tomography in children and young adults with congenital heart disease: comparison with lung perfusion scintigraphy. Pediatr Radiol 47:1580–1587
Goo HW (2018) Computed tomography pulmonary vascular volume ratio in children and young adults with congenital heart disease: the effect of cardiac phase. Pediatr Radiol 48:915–922
Goo HW (2019) Computed tomography pulmonary vascular volume ratio can be used to evaluate the effectiveness of pulmonary angioplasty in peripheral pulmonary artery stenosis. Korean J Radiol 20:1422–1430
Goo HW, Park SH (2020) Optimal attenuation threshold for quantifying CT pulmonary vascular volume ratio. Korean J Radiol 21:756–763
Goo HW, Park SH (2022) Prediction of pulmonary hypertension using central-to-peripheral pulmonary vascular volume ratio on three-dimensional cardiothoracic CT in patients with congenital heart disease. Jpn J Radiol 40:961–969
Vida VL, Rito ML, Zucchetta F et al (2013) Pulmonary artery branch stenosis in patients with congenital heart disease. J Card Surg 28:439–445
Pruckmayer M, Zacherl S, Salzer-Muhar U et al (1999) Scintigraphic assessment of the pulmonary and whole-body blood flow patterns after surgical intervention in congenital heart disease. J Nucl Med 40:1477–1483
Rengier F, Melzig C, Derlin T et al (2019) Advanced imaging in pulmonary hypertension: emerging techniques and applications. Int J Cardiovasc Imaging 35:1407–1420
Rengier F, Wörz S, Melzig C et al (2016) Automated 3D volumetry of the pulmonary arteries based on magnetic resonance angiography has potential for predicting pulmonary hypertension. PLoS ONE 11:e0162516
Melzig C, Wörz S, Egenlauf B et al (2019) Combined automated 3D volumetry by pulmonary CT angiography and echocardiography for detection of pulmonary hypertension. Eur Radiol 29:6059–6068
Rahaghi FN, Ross JC, Agarwal M et al (2016) Pulmonary vascular morphology as an imaging bio marker in chronic thromboembolic pulmonary hypertension. Pulm Circ 6:70–81
Alnasser TN, Abdulaal L, Maiter A et al (2024) Advancements in cardiac structures segmentation: a comprehensive systematic review of deep learning in CT imaging. Front Cardiovasc Med 11:1323461
Goo HW, Park SH (2023) Partial voxel interpolation to reduce partial volume error of cardiac computed tomography ventricular volumetry in patients with congenital heart disease. Pediatr Radiol 53:2528–2538
Dini FL, Guarini G, Ballo P et al (2013) The left ventricle as a mechanical engine: from Leonardo da Vinci to the echocardiographic assessment of peak power output-to-left ventricular mass. J Cardiovasc Med (Hagerstown) 14:214–220
Xu S, Zhang Z, Zhou Q et al (2021) A pulmonary vascular extraction algorithm from chest CT/CTA images. J Healthc Eng 2021:5763177
Nam JG, Witanto JN, Park SJ et al (2021) Automatic pulmonary vessel segmentation on noncontrast chest CT: deep learning algorithm developed using spatiotemporally matched virtual noncontrast images and low-keV contrast-enhanced vessel maps. Eur Radiol 31:9012–9021
Pan L, Yan X, Zheng Y et al (2023) Automatic pulmonary artery-vein separation in CT images using a twin-pipe network and topology reconstruction. PeerJ Comput Sci 9:e1537
Goo HW, Park SJ, Yoo SJ (2020) Advanced medical use of three-dimensional imaging in congenital heart disease: augmented reality, mixed reality, virtual reality, and three-dimensional printing. Korean J Radiol 21:133–145
Author information
Authors and Affiliations
Contributions
All authors whose names appear on the submission:
(1) made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data;
(2) drafted the work or revised it critically for important intellectual content;
(3) approved the version to be published; and.
(4) agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goo, H.W. Pediatric three-dimensional quantitative cardiovascular computed tomography. Pediatr Radiol (2024). https://doi.org/10.1007/s00247-024-05931-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00247-024-05931-7