Abstract
Background
Early precision diagnosis and effective treatment of opsoclonus myoclonus ataxia syndrome (OMAS) patients presenting with neuroblastoma can prevent serious neurological outcomes.
Objective
To assess the diagnostic value of 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) imaging in pediatric OMAS with neuroblastoma.
Materials and methods
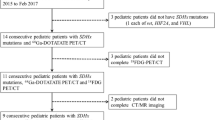
A retrospective evaluation of 45 patients diagnosed with OMAS who underwent 18F-FDG PET/CT was performed. A univariate analysis was performed to compare clinical characteristics between OMAS with and without neuroblastoma. Univariate and multivariate logistic regression analyses were applied to identify independent risk factors for OMAS with neuroblastoma and to develop the clinical model. Finally, independent risk factors and PET/CT were fitted to build the combined model for the diagnosis of OMAS with neuroblastoma and presented as a nomogram. Receiver operating characteristic curve, decision curve, and calibration curve analyses were conducted to evaluate the performance of the models.
Results
Among 45 patients, 27 were PET/CT-positive, 23/27 lesions were neuroblastoma, and four were false positives. One of the false positive patients was confirmed to be adrenal reactive hyperplasia by postoperative pathology, and the symptoms of OMAS disappeared in the remaining three cases during clinical follow-up. The average maximal standardized uptake value of PET/CT-positive lesions was 2.6. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of PET/CT were 100%, 81.8%, 85.2%, 100%, and 91.1%, respectively. Age at diagnosis, lactate dehydrogenase, and neuron-specific enolase showed statistically significant differences between OMAS with and without neuroblastoma. Lactate dehydrogenase was identified as the independent risk factor to develop the clinical model, and the clinical model demonstrated an area under the curve (AUC) of 0.82 for the diagnosis of OMAS with neuroblastoma, with an AUC as high as 0.91 when combined with PET/CT. The decision curve analysis and calibration curve demonstrated that the nomogram had good consistency and clinical usefulness.
Conclusion
In patients with OMAS, 18F-FDG PET/CT has a high diagnostic accuracy in detecting tumors of the neuroblastoma, especially when combined with the independent risk factor serum lactate dehydrogenase.
Graphical abstract







Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding authors on reasonable request.
References
Kinsbourne M (1962) Myoclonic encephalopathy of infants. J Neurol Neurosurg Psychiatry 25:271–276
Rudnick E, Khakoo Y, Antunes NL et al (2001) Opsoclonus-myoclonus-ataxia syndrome in neuroblastoma: clinical outcome and antineuronal antibodies-a report from the children’s Cancer Group Study. Med Pediatr Oncol 36:612–622
Hasegawa S, Matsushige T, Kajimoto M et al (2015) A nationwide survey of opsoclonus–myoclonus syndrome in Japanese children. Brain Dev 37:656–660
Bar-Sever Z, Biassoni L, Shulkin B et al (2018) Guidelines on nuclear medicine imaging in neuroblastoma. Eur J Nucl Med Mol Imaging 45:2009–2024
Rossor T, Yeh EA, Khakoo Y et al (2022) Diagnosis and management of opsoclonus-myoclonus-ataxia syndrome in children. Neurol Neuroimmunol Neuroinflamm 9:e1153
Kumar R, Vankadari K, Mittal BR et al (2021) Diagnostic values of 68Ga-labelled DOTANOC PET/CT imaging in pediatric patients presenting with paraneoplastic opsoclonus myoclonus ataxia syndrome. Eur Radiol 31:4587–4594
Biasotti S, Garaventa A, Villavecchia GP et al (2000) False-negative metaiodobenzylguanidine scintigraphy at diagnosis of neuroblastoma. Med Pediatr Oncol 35:153–155
Brunklaus A, Pohl K, Zuberi SM, de Sousa C (2012) Investigating neuroblastoma in childhood opsoclonus-myoclonus syndrome. Arch Dis Child 97:461–463
Sharp SE, Shulkin BL, Gelfand MJ et al (2009) 123I-MIBG scintigraphy and 18F-FDG PET in neuroblastoma. J Nucl Med 50:1237–1243
Melzer HI, Coppenrath E, Schmid I et al (2011) 123I-MIBG scintigraphy/SPECT versus 18F-FDG PET in paediatric neuroblastoma. Eur J Nucl Med Mol Imaging 38:1648–1658
Feng L, Li S, Wang C, Yang J (2023) Current status and future perspective on molecular imaging and treatment of neuroblastoma. Semin Nucl Med 53:517–529
Bleeker G, Tytgat GA, Adam JA et al (2015) 123I-MIBG scintigraphy and 18F-FDG-PET imaging for diagnosing neuroblastoma. Cochrane Database Syst Rev:CD009263
Li C, Zhang J, Chen S et al (2018) Prognostic value of metabolic indices and bone marrow uptake pattern on preoperative 18F-FDG PET/CT in pediatric patients with neuroblastoma. Eur J Nucl Med Mol Imaging 45:306–315
Bar-Sever Z, Keidar Z, Ben-Barak A et al (2007) The incremental value of 18F-FDG PET/CT in paediatric malignancies. Eur J Nucl Med Mol Imaging 34:630–637
Joshi P, Lele V (2013) Somatostatin receptor positron emission tomography/computed tomography (PET/CT) in the evaluation of opsoclonus-myoclonus ataxia syndrome. Indian J Nucl Med 28:108–111
Matthay KK, Blaes F, Hero B et al (2005) Opsoclonus myoclonus syndrome in neuroblastoma a report from a workshop on the dancing eyes syndrome at the advances in neuroblastoma meeting in Genoa, Italy, 2004. Cancer Lett 228:275–282
Pranzatelli MR, Tate ED, McGee NR (2017) Demographic, clinical, and immunologic features of 389 children with opsoclonus-myoclonus syndrome: a cross-sectional study. Front Neurol 8:468
Ki Pang K, de Sousa C, Lang B, Pike MG (2010) A prospective study of the presentation and management of dancing eye syndrome/opsoclonus–myoclonus syndrome in the United Kingdom. Eur J Paediatr Neurol 14:156–161
Blaes F, Dharmalingam B (2016) Childhood opsoclonus-myoclonus syndrome: diagnosis and treatment. Expert Rev Neurother 16:641–648
Russo C, Cohn SL, Petruzzi MJ, de Alarcon PA (1997) Long-term neurologic outcome in children with opsoclonus-myoclonus associated with neuroblastoma: a report from the Pediatric Oncology Group. Med Pediatr Oncol 28:284–288
Koh PS, Raffensperger JG, Berry S et al (1994) Long-term outcome in children with opsoclonus-myoclonus and ataxia and coincident neuroblastoma. J Pediatr 125:712–716
Mitchell WG, Davalos-Gonzalez Y, Brumm VL et al (2002) Opsoclonus-ataxia caused by childhood neuroblastoma: developmental and neurologic sequelae. Pediatrics 109:86–98
Hayward K, Jeremy RJ, Jenkins S et al (2001) Long-term neurobehavioral outcomes in children with neuroblastoma and opsoclonus-myoclonus-ataxia syndrome: relationship to MRI findings and anti-neuronal antibodies. J Pediatr 139:552–559
Martiniova L, Perera SM, Brouwers FM et al (2011) Increased uptake of [123I]meta-iodobenzylguanidine, [18F]fluorodopamine, and [3H]norepinephrine in mouse pheochromocytoma cells and tumors after treatment with the histone deacetylase inhibitors. Endocr Relat Cancer 18:143–157
Rothenberg AB, Berdon WE, D’Angio GJ et al (2009) The association between neuroblastoma and opsoclonus-myoclonus syndrome: a historical review. Pediatr Radiol 39:723–726
Cohn SL, Pearson AD, London WB et al (2009) The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 27:289–297
Altman AJ, Baehner RL (1976) Favorable prognosis for survival in children with coincident opso-myoclonus and neuroblastoma. Cancer 37:846–852
Simon T, Hero B, Hunneman DH, Berthold F (2003) Tumour markers are poor predictors for relapse or progression in neuroblastoma. Eur J Cancer 39:1899–1903
Funding
This work was supported by the National Natural Science Foundation of China (No. 82272034).
Author information
Authors and Affiliations
Contributions
Conceptualization: L.F., S.Y., H.W., and J.Y.; data curation: L.F., S.Y., and Y.L.; formal analysis: J.L., Z.C., and Q.Z.; funding acquisition: J.Y.; investigation: S.Y. and Y.L.; methodology: L.F., S.Y., and Y.L.; project administration: H.W. and J.Y.; resources: H.W. and J.Y.; software: L.F., S.Y., and J.L.; supervision: H.W. and J.Y.; validation: L.F., S.Y., J.L., Z.C., and Q.Z.; visualization: Y.L., J.L., Z.C., Q.Z., and Q.Z.; writing — original draft preparation: L.F., S.Y., Y.L., J.L., Z.C., Q.Z., H.W., and J.Y.; writing — review and editing: L.F., S.Y., Y.L., J.L., Z.C., Q.Z., H.W., and J.Y. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of Beijing Friendship Hospital, Capital Medical University.
Informed consent
The requirement to obtain informed consent was waived by the Institutional Review Board due to the use of retrospective anonymized data.
Conflicts of interest
None
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, L., Yang, S., Lin, Y. et al. Diagnostic value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography imaging in pediatric opsoclonus myoclonus ataxia syndrome presenting with neuroblastoma. Pediatr Radiol (2024). https://doi.org/10.1007/s00247-024-05921-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00247-024-05921-9