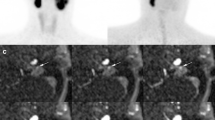
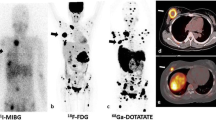
Abstract
Purpose
To evaluate and compare diagnostic performance of 68Ga-DOTA(0)-Tyr(3)-octreotate (68Ga-DOTATATE) with 18F–fluoro-2-deoxy-D-glucose (18F–FDG) positron emission tomography-computed tomography (PET/CT) and anatomic imaging using computed tomography and/or magnetic resonance (CT/MR) imaging in detection of SDHx-related pheochromocytomas and paragangliomas (PPGLs) in pediatric patients.
Methods
Nine pediatric patients (5:4, girls:boys; 14.6 ± 2.0 years) with an SDHx-related mutation (SDHB:SDHA:SDHD, n = 7:1:1) were included in this retrospective study. At the time of initial diagnosis, 7/9 patients had metastatic disease. They underwent CT/MR imaging along with PET/CT using 68Ga-DOTATATE (n = 9), 18F–FDG (n = 8), and positron emission tomography-magnetic resonance imaging (PET/MR) using 18F–FDG (n = 1). In this manuscript, 18F–FDG PET/CT refers to both 18F–FDG PET/CT and 18F–FDG PET/MR. The per-lesion, per-region, and per-patient detection rates were compared and calculated for each of the imaging modalities. A composite of all functional and anatomic imaging studies served as the imaging comparator.
Results
Eight out of nine patients were positive for PPGLs on the imaging studies that demonstrated 107 lesions in 22 anatomic regions on the imaging comparator. The per-lesion detection rates for 68Ga-DOTATATE PET/CT, 18F–FDG PET/CT, and CT/MR imaging were 93.5% (95%CI, 87.0% to 97.3%); 79.4% (95%CI, 70.5% to 86.6%); and 73.8% (95%CI, 64.5% to 81.9%), respectively. The per-lesion detection rate for 68Ga-DOTATATE PET/CT was significantly higher than that of 18F–FDG PET/CT (p = 0.001) or CT/MR imaging (p < 0.001). In all of the anatomic regions except abdomen, the per-lesion detection rates for 68Ga-DOTATATE PET/CT was found to be equal or superior to 18F–FDG PET/CT, and CT/MR imaging. The per-region detection rate was 100% (95%CI, 84.6% to 100%) for 68Ga-DOTATATE PET/CT and 90.9% (95%CI, 70.8% to 98.9%) for both 18F–FDG PET/CT and CT/MR imaging. The per-patient detection rates for 68Ga-DOTATATE PET/CT, 18FDG PET/CT, and CT/MR imaging were all 100% (95%CI, 63.1% to 100%).
Conclusion
Our preliminary study demonstrates the superiority of 68Ga-DOTATATE PET/CT in localization of SDHx-related PPGLs in pediatric population compared to 18F–FDG PET/CT and CT/MR imaging with the exception of abdominal (excluding adrenal and liver) lesions, and suggests that it might be considered as a first-line imaging modality in pediatric patients with SDHx-related PPGLs.



Similar content being viewed by others
References
Schiffman JD. No child left behind in SDHB testing for paragangliomas and pheochromocytomas. J Clin Oncol. 2011;29:4070–2. https://doi.org/10.1200/JCO.2011.37.8695.
Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346:1459–66. https://doi.org/10.1056/NEJMoa020152.
Neumann HP, Pawlu C, Peczkowska M, Bausch B, McWhinney SR, Muresan M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292:943–51. https://doi.org/10.1001/jama.292.8.943.
Burnichon N, Briere JJ, Libe R, Vescovo L, Riviere J, Tissier F, et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19:3011–20. https://doi.org/10.1093/hmg/ddq206.
Bayley JP, Kunst HP, Cascon A, Sampietro ML, Gaal J, Korpershoek E, et al. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol. 2010;11:366–72. https://doi.org/10.1016/S1470-2045(10)70007-3.
Michalowska I, Cwikla JB, Peczkowska M, Furmanek MI, Buscombe JR, Michalski W, et al. Usefulness of somatostatin receptor scintigraphy (Tc-[HYNIC, Tyr3]-octreotide) and 123I-Metaiodobenzylguanidine scintigraphy in patients with SDHx gene-related Pheochromocytomas and Paragangliomas detected by computed tomography. Neuroendocrinology. 2015;101:321–30. https://doi.org/10.1159/000381458.
Bezawork-Geleta A, Rohlena J, Dong L, Pacak K, Neuzil J. Mitochondrial Complex II: At the Crossroads. Trends Biochem Sci. 2017;42:312–325. doi:https://doi.org/10.1016/j.tibs.2017.01.003.
Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011;96:717–25. https://doi.org/10.1210/jc.2010-1946.
Turkova H, Prodanov T, Maly M, Martucci V, Adams K, Widimsky J Jr, et al. Characteristics and outcomes of metastatic Sdhb and sporadic Pheochromocytoma/Paraganglioma: an National Institutes of Health study. Endocr Pract. 2016;22:302–14. https://doi.org/10.4158/EP15725.OR.
Benn DE, Gimenez-Roqueplo AP, Reilly JR, Bertherat J, Burgess J, Byth K, et al. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab. 2006;91:827–36. https://doi.org/10.1210/jc.2005-1862.
Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92:3822–8. https://doi.org/10.1210/jc.2007-0709.
Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Eisenhofer G, et al. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. J Natl Cancer Inst. 2012;104:700–8. https://doi.org/10.1093/jnci/djs188.
Parenti G, Zampetti B, Rapizzi E, Ercolino T, Giache V, Mannelli M. Updated and new perspectives on diagnosis, prognosis, and therapy of malignant pheochromocytoma/paraganglioma. J Oncol. 2012;2012:872713. https://doi.org/10.1155/2012/872713.
Babic B, Patel D, Aufforth R, Assadipour Y, Sadowski SM, Quezado M, et al. Pediatric patients with pheochromocytoma and paraganglioma should have routine preoperative genetic testing for common susceptibility genes in addition to imaging to detect extra-adrenal and metastatic tumors. Surgery. 2017;161:220–7. https://doi.org/10.1016/j.surg.2016.05.059.
Edmonds S, Fein DM, Gurtman A. Pheochromocytoma. Pediatr Rev. 2011;32:308–10. https://doi.org/10.1542/pir.32-7-308.
Ciftci AO, Tanyel FC, Senocak ME, Buyukpamukcu N. Pheochromocytoma in children. J Pediatr Surg. 2001;36(3):447–52. https://doi.org/10.1053/jpsu.2001.21612.
Barontini M, Levin G, Sanso G. Characteristics of pheochromocytoma in a 4- to 20-year-old population. Ann N Y Acad Sci. 2006;1073:30–7. https://doi.org/10.1196/annals.1353.003.
King KS, Prodanov T, Kantorovich V, Fojo T, Hewitt JK, Zacharin M, et al. Metastatic pheochromocytoma/paraganglioma related to primary tumor development in childhood or adolescence: significant link to SDHB mutations. J Clin Oncol. 2011;29:4137–42. https://doi.org/10.1200/JCO.2011.34.6353.
Robbins E. Radiation risks from imaging studies in children with cancer. Pediatr Blood Cancer. 2008;51:453–7. https://doi.org/10.1002/pbc.21599.
Fahey FH, Treves ST, Adelstein SJ. Minimizing and communicating radiation risk in pediatric nuclear medicine. J Nucl Med. 2011;52:1240–51. https://doi.org/10.2967/jnumed.109.069609.
Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–42. https://doi.org/10.1210/jc.2014-1498.
Janssen I, Blanchet EM, Adams K, Chen CC, Millo CM, Herscovitch P, et al. Superiority of [68Ga]-DOTATATE PET/CT to other functional imaging modalities in the localization of SDHB-associated metastatic Pheochromocytoma and Paraganglioma. Clin Cancer Res. 2015;21:3888–95. https://doi.org/10.1158/1078-0432.CCR-14-2751.
U.S. Food and Drug Administration. FDA approves new diagnostic imaging agent to detect rare neuroendocrine tumors. 2016. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm504524.htm. Accessed 05/30/2017.
Reubi JC, Waser B, Schaer JC, Laissue JA. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med. 2001;28:836–46.
Reubi JC, Schar JC, Waser B, Wenger S, Heppeler A, Schmitt JS, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273–82.
Janssen I, Chen CC, Millo CM, Ling A, Taieb D, Lin FI, et al. PET/CT comparing (68)Ga-DOTATATE and other radiopharmaceuticals and in comparison with CT/MRI for the localization of sporadic metastatic pheochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2016;43:1784–91. https://doi.org/10.1007/s00259-016-3357-x.
Janssen I, Chen CC, Taieb D, Patronas NJ, Millo CM, Adams KT, et al. 68Ga-DOTATATE PET/CT in the localization of head and neck Paragangliomas compared with other functional imaging modalities and CT/MRI. J Nucl Med. 2016;57:186–91. https://doi.org/10.2967/jnumed.115.161018.
Kong G, Grozinsky-Glasberg S, Hofman MS, Callahan J, Meirovitz A, Maimon O, et al. Efficacy of peptide receptor radionuclide therapy (PRRT) for functional metastatic Paraganglioma and Phaeochromocytoma. J Clin Endocrinol Metab. 2017; https://doi.org/10.1210/jc.2017-00816.
Favier J, Briere JJ, Burnichon N, Riviere J, Vescovo L, Benit P, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4:e7094. https://doi.org/10.1371/journal.pone.0007094.
Biermann M, Schwarzlmuller T, Fasmer KE, Reitan BC, Johnsen B, Rosendahl K. Is there a role for PET-CT and SPECT-CT in pediatric oncology? Acta Radiol. 2013;54:1037–45. https://doi.org/10.1258/ar.2012.120616.
Culverwell AD, Scarsbrook AF, Chowdhury FU. False-positive uptake on 2-[(1)(8)F]-fluoro-2-deoxy-D-glucose (FDG) positron-emission tomography/computed tomography (PET/CT) in oncological imaging. Clin Radiol. 2011;66:366–82. https://doi.org/10.1016/j.crad.2010.12.004.
Acknowledgements
We would like to thank Dr. Paul Wakim for the assistance in statistical analyses. We would also like to thank the patients and their families, for participating in the study, and all of the people who participated in this project, especially the technologists in the NIH Clinical Center PET Department.
Financial disclosure
This work was supported, in part, by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development and was supported, in part, by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by National Institutes of Health (grant number: Z1AHD008735).
Conflict of interest
Author Abhishek Jha declares no conflict of interest. Author Alexander Ling declares no conflict of interest. Author Corina Millo declares no conflict of interest. Author Garima Gupta declares no conflict of interest. Author Bruna Viana declares no conflict of interest. Author Frank I. Lin declares no conflict of interest. Author Peter Herscovitch declares no conflict of interest. Author Karen T. Adams declares no conflict of interest. Author David Taïeb declares no conflict of interest. Author Adam R. Metwalli declares no conflict of interest. Author W. Marston Linehan declares no conflict of interest. Author Alessandra Brofferio declares no conflict of interest. Author Constantine A. Stratakis declares no conflict of interest. Author Electron Kebebew declares no conflict of interest. Author Maya Lodish declares no conflict of interest. Author Ali Cahid Civelek declares no conflict of interest. Author Karel Pacak declares no conflict of interest. Informed consent was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed assent from the patients and signed permission from one or both parents were obtained for all the individual participants included in the study.
Additional information
Ali Cahid Civelek and Karel Pacak equally share the senior authorship.
Rights and permissions
About this article
Cite this article
Jha, A., Ling, A., Millo, C. et al. Superiority of 68Ga-DOTATATE over 18F-FDG and anatomic imaging in the detection of succinate dehydrogenase mutation (SDHx )-related pheochromocytoma and paraganglioma in the pediatric population. Eur J Nucl Med Mol Imaging 45, 787–797 (2018). https://doi.org/10.1007/s00259-017-3896-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3896-9