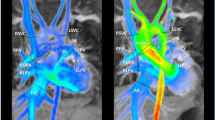
Abstract
Background
Ferumoxytol is becoming more widely used as an off-label blood-pool contrast agent for MR angiography (MRA) and four-dimensional (4D) flow imaging in pediatric cardiovascular disease. Brand and generic versions of ferumoxytol are available with no information on relative efficacy as a contrast agent and safety profiles.
Objective
This study evaluates patient safety and image quality of comparable dosages of generic ferumoxytol (GF) versus brand ferumoxytol (BF) with the following hypotheses: (1) Reducing the contrast dosage from 3 to 2 mg/kg will not affect imaging quality and diagnostic accuracy of MRA and four-dimensional 4D flow. (2) GF and BF have similar image quality. (3) GF and BF have similar patient safety profiles.
Materials and methods
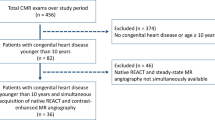
In an IRB-approved retrospective study, changes in vitals/clinical status between baseline, during infusion, and 30 min post-infusion were analyzed in 3 groups: group 1 (3 mg/kg BF, 216 patients, age: 19.29 ± 11.71 years ranging from 2 months to 62 years), group 2 (2 mg/kg BF, 47 patients, age: 15.35 ± 8.56 years ranging from 10 days to 41 years), and group 3 (2 mg/kg GF, 127 patients, age: 17.16 ± 12.18 years ranging from 6 days to 58 years). Both pediatric and adult patients with congenital heart disease (CHD) indications were included within the study. Adverse reactions were classified as mild, moderate, or severe. Quantitative analysis of MR image quality was performed with signal-to-noise ratio (SNR) on MRA and velocity-to-noise ratio (VNR) on 4D flow. Qualitative grading of imaging features was performed by 2 experienced observers. Two-way analysis of variance (ANOVA) and chi-square tests were used for comparison with a P value of ≤ 0.05 used for significance.
Results
No statistical difference was found in clinical status and vital signs (P>0.05). No severe reactions were reported. 7.9% of GF patients experienced an adverse reaction compared to 2.3% with 3 mg/kg BF and 8.4% with 2 mg/kg BF. There was no statistical difference in SNR between the 3 groups (P>0.05). For 4D flow, 2 mg/kg GF demonstrated an increase in VNR compared to 2 mg/kg BF (P = 0.005). The qualitative scores for MRA and 4D flow were high (≥ 3) across all 3 groups.
Conclusions
No significant difference was identified between 2 mg/kg GF and BF in terms of safety profile and image quality. Given the small sample size of this study, further studies are required to confirm these results.
Graphical Abstract


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author (RK) upon reasonable request.
References
Jin R, Lin B, Li D, Ai H (2014) Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: design considerations and clinical applications. Curr Opin Pharmacol 18:18–27
Corot C, Robert P, Idée JM, Port M (2006) Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev 58:1471–1504
Balakrishnan VS, Rao M, Kausz AT et al (2009) Physicochemical properties of ferumoxytol, a new intravenous iron preparation. Eur J Clin Invest 39:489–496
Toth GB, Varallyay CG, Horvath A et al (2017) Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int 92:47–66
Ruangwattanapaisarn N, Hsiao A, Vasanawala SS (2015) Ferumoxytol as an off-label contrast agent in body 3T MR angiography: a pilot study in children. Pediatr Radiol 45:831–839
Cheng JY, Hanneman K, Zhang T et al (2016) Comprehensive motion-compensated highly accelerated 4D flow MRI with ferumoxytol enhancement for pediatric congenital heart disease. J Magn Reson Imaging 43:1355–1368
Kowalczyk M, Banach M, Rysz J (2011) Ferumoxytol: a new era of iron deficiency anemia treatment for patients with chronic kidney disease. J Nephrol 24:717–722
Hetzel D, Strauss W, Bernard K, Li Z, Urboniene A, Allen LF (2014) A Phase III, randomized, open-label trial of ferumoxytol compared with iron sucrose for the treatment of iron deficiency anemia in patients with a history of unsatisfactory oral iron therapy. Am J Hematol 89:646–650
Finn JP, Nguyen KL, Han F et al (2016) Cardiovascular MRI with ferumoxytol. Clin Radiol 71:796–806
Pai AB, Garba AO (2012) Ferumoxytol: a silver lining in the treatment of anemia of chronic kidney disease or another dark cloud? J Blood Med 3:77–85
Food & Drug Administration. FDA Drug Safety Communication: FDA strengthens warnings and changes prescribing instructions to decrease the risk of serious allergic reactions with anemia drug Feraheme (ferumoxytol). Available: http://www.fda.gov/Drugs/DrugSafety/ucm440138.htm. Accessed 28 Sept 2023
Nguyen KL, Yoshida T, Kathuria-Prakash N et al (2019) The rise of off-label iron-based agents in magnetic resonance imaging. J Radiol Nurs 38:https://doi.org/10.1016/j.jradnu.2018.11.004
Vasanawala SS, Nguyen KL, Hope MD et al (2016) Safety and technique of ferumoxytol administration for MRI. Magn Reson Med 75:2107–2111
Dahl NV, Kaper RF, Strauss WE, Corvino FA, Zivkovic M (2017) Cost-effectiveness analysis of intravenous ferumoxytol for the treatment of iron deficiency anemia in adult patients with non-dialysis-dependent chronic kidney disease in the USA. Clinicoecon Outcomes Res 9:557–567
Lim W, Afif W, Knowles S et al (2019) Canadian expert consensus: management of hypersensitivity reactions to intravenous iron in adults. Vox Sang 114:363–373
2021 ACR Manual on Contrast Media. 10.3. American College of Radiology, pp 100–101. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed 28 Sept 2023
Rampton D, Folkersen J, Fishbane S et al (2014) Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica 99:1671–1676
Nilsson A, Markenroth Bloch K et al (2012) Variable velocity encoding in a three-dimensional, three-directional phase contrast sequence: evaluation in phantom and volunteers. J Magn Reson Imaging 36:1450–1459
Zhou Z, Han F, Rapacchi S et al (2017) Accelerated ferumoxytol-enhanced 4D multiphase, steady-state imaging with contrast enhancement (MUSIC) cardiovascular MRI: validation in pediatric congenital heart disease. NMR Biomed 30
Kanda T, Oba H, Toyoda K, Kitajima K, Furui S (2016) Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn J Radiol 34:3–9
Storey P, Lim RP, Chandarana H et al (2012) MRI assessment of hepatic iron clearance rates after USPIO administration in healthy adults. Invest Radiol 47:717–724
Worm M, Francuzik W, Renaudin J-M et al (2018) Factors increasing the risk for a severe reaction in anaphylaxis: an analysis of data from The European Anaphylaxis Registry. Allergy 73:1322–1330
Assessment report for: Iron containing intravenous (IV) medicinal products. (2013) EMA/549569/2013
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declarations
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. IRB approval was obtained for this retrospective study.
Conflicts of interest
None
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dasi, A., Kring, D.N., Selvaraj, B. et al. Brand ferumoxytol vs. generic ferumoxytol comparison across two dosing regimens: a cardiac MRI image quality study. Pediatr Radiol 53, 2622–2632 (2023). https://doi.org/10.1007/s00247-023-05778-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-023-05778-4