Abstract
Background
Aside from single-center reports, few data exist across pediatric institutions that examine overall MRI turnaround time (TAT) and the determinants of variability.
Objective
To determine average duration and determinants of a brain MRI examination at academic pediatric institutions and compare the duration to those used in practice expense relative value units (RVUs).
Materials and methods
This multi-institutional cross-sectional investigation comprised four academic pediatric hospitals. We included children ages 0 to < 18 years who underwent an outpatient MRI of the brain without contrast agent in 2019. Our outcome of interest was the overall MRI TAT derived by time stamps. We estimated determinants of overall TAT using an adjusted log-transformed multivariable linear regression model with robust standard errors.
Results
The average overall TAT significantly varied among the four hospitals. A sedated brain MRI ranged from 158 min to 224 min, a non-sedated MRI from 70 min to 112 min, and a limited MRI from 44 min to 70 min. The most significant predictor of a longer overall TAT was having a sedated MRI (coefficient = 0.71, 95% confidence interval [CI]: 0.66–0.75; P < 0.001). The median MRI scan time for a non-sedated exam was 38 min and for a sedated exam, 37 min, approximately double the duration used by the Relative Value Scale (RVS) Update Committee (RUC).
Conclusion
We found considerable differences in the overall TAT across four pediatric academic institutions. Overall, the significant predictors of turnaround times were hospital site and MRI pathway (non-sedated versus sedated versus limited MRI).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For many neurologic indications, MRI is the preferred imaging examination because of its superior tissue resolution and because it avoids ionizing radiation [1]. One key disadvantage of MRI, particularly when compared to CT, is the considerably longer examination duration. For institutions that rely on the same scanners for both inpatients and outpatients, the longer examinations can lead to bottlenecks in the patient care pathway, delaying discharge and increasing length of stay for hospitalized patients [1, 2]. From an operational perspective, optimizing efficient scheduling of imaging examinations requires an accurate measurement of MRI examination length. In the pediatric setting, some children are too young to tolerate and remain motionless for an MRI examination, necessitating the use of sedation or general anesthesia, which can extend examination times [3].
Imaging duration is a factor accounted for in practice expense relative value unit (RVU) valuation and reimbursement. These durations were established by the American Medical Association (AMA)’s specialty society Relative Value Scale Update Committee (RUC). The RUC formulates recommendations to the Centers for Medicare & Medicaid Services (CMS) regarding RVU values for new medical services and periodically updates these values for existing medical services. The RUC’s current approach in estimating imaging durations is through surveys, where radiologists are presented vignettes and asked to estimate the amount of time it would take to perform the imaging study undergoing valuation. Studies have shown that relying on physician surveys can result in biased estimates (both over- and underestimates) of examination duration [4]. Additionally, the RVU valuation might not be tailored to neonates and children, although it is hypothesized that examinations in this population can take longer because of motion, and these longer examinations result in an undervaluation of RVUs in the pediatric population when compared to the adult setting [5]. Because of this particular limitation, the American Academy of Pediatrics (AAP) [6] has advocated that time estimates be tailored to account for pediatric examinations to ensure appropriate RVU assignments [7,8,9]; however, the appropriate allocation of RVUs requires empirical benchmarking of examination duration from multiple institutions.
Single-center reports have depicted patient MRI flow and turnaround time (TAT) [10,11,12]. However, few data across pediatric institutions examine MRI overall TAT, MRI acquisition duration, and the determinants of duration variability. A more comprehensive understanding of overall TAT in pediatric MRIs could allow for future benchmarking of timeliness in the pediatric setting. The primary aim of this study was descriptive and was to accurately determine the average duration of a brain MRI examination at academic pediatric institutions. Our secondary aim was to identify the determinants that contribute to duration variance in brain MRI examination. Third, because other reports have found that the time estimates for RVUs are biased, we compared the average brain MRI durations to the time durations used in practice expense RVUs.
Materials and methods
This multi-institutional cross-sectional investigation comprised a convenience sample of four academic pediatric hospitals. Hospitals were included based on access to data, geographic diversity within the United States and nonprofit academic status. We controlled for broad health care quality by selecting hospitals that were ranked in the top 10% of pediatric hospitals for 2020–2021 in the U.S. News & World Report (USNWR) [13]. Additionally, because the specialty of pediatric radiology is not specifically ranked by USNWR, we relied on the report’s overall institutional rankings. We obtained local institutional review board (IRB) approval at each hospital. Patient informed consent was waived because the study was retrospective, with no direct patient contact.
Study population
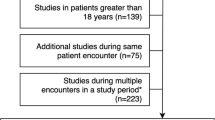
We identified children for the study through the four institutions’ electronic medical record (EMR) systems. We included children ages 0 to < 18 years who underwent outpatient non-contrast MRI of the brain in 2019. We specifically chose 2019 to avoid potential bias caused by the coronavirus disease 2019 (COVID-19), which affected workflows and examination durations at U.S. hospitals primarily in the spring of 2020 [14]. We included children who underwent an MRI of the brain with sedation or general anesthesia (referred to as “sedated MRI” in this study); a non-sedated MRI of the brain; or a limited MRI of the brain consisting of an abbreviated protocol, commonly with three sequences and billed with a limited modifier [15, 16]. We excluded brain MRI examinations that were combined with other imaging examinations because this was expected to overestimate TAT.
Variables
Our main outcome of interest was the overall MRI TAT derived by time stamps recorded in the electronic medical records. For sedated examinations, the overall MRI TAT was calculated starting from patient check-in with registration and ending upon patient discharge from the post-anesthesia care unit (PACU). For non-sedated and limited MRI examinations, we calculated the overall MRI TAT starting from patient check-in and ending with the conclusion of the MRI scan. Because our focus was on the patient examination duration, we excluded the radiologist interpretation time. From the EMR time stamps, we divided the MRI process into four workflow phases consisting of (1) the child’s wait time after registration/arrival, (2) the MRI pre-procedure phase, (3) the MRI acquisition and (4) post-procedure care (only for sedated examinations).
We selected independent predictors based on the literature [12] and included hospital, MRI pathway type (sedated versus non-sedated versus limited MRI) and patient-level predictors of age and gender. A list of MRI sequences for both non-contrast standard and limited MRI of the brain is shown in Table 1, though we did not include this as a predictor because of its multicollinearity with the hospital indicator variable.
Sampling
We used a sampling approach to facilitate data collection. Where it was feasible, institutions provided an automated detailed report, inclusive of patient and time stamp variables. However, at two institutions an automated report was not feasible, and we acquired the data by manually reviewing patient charts. To decrease this manual data collection burden, we used a sampling strategy. Sampling consisted of the following steps: First, we obtained an exhaustive list of patients from each institution’s EMR who met our inclusion criteria. We then obtained a simple random sample (using statistical software) of n = 343 children per hospital, for a total combined sample of 1,372 children across the four hospitals.
Our sample size determination was driven by our primary aim of benchmarking TAT in each category of brain MRI (non-sedated, sedated and limited), where we treated each MRI type as a single group for precision analysis. Power was based on a descriptive one-sample mean on the expected mean overall TAT from a previous study [12]; we estimated a standard deviation of 49 min for non-sedated awake patients and 68 min for sedated MRIs, with a maximum effect size of 5%. For the non-sedated MRI brain category, this calculation suggested a sample size of 105 to estimate the overall TAT within a 5% precision or effect size with 95% confidence (for each institution). For sedated brain MRIs, this calculation suggested a sample size of 188 to estimate the overall TAT within a 5% precision or effect size with 95% confidence (for each institution). We also included 50 limited MRIs from each hospital. We estimated a small variation for these abbreviated examination types because previous literature estimated the scan length to be less than 5 min [16]. Overall, our sampling resulted in utilizing 14–24% of the hospitals’ examinations based on their annual volumes: hospital A, 22% (343/1,573); hospital B, 24% (343/1,455); hospital C, 18% (343/1,928); hospital D, 14% (343/2,422).
Statistical analysis
All analyses were completed in Stata version 17.0 (StataCorp LP, College Station, TX).
We used the Kruskal–Wallis to compare our main outcome variable, overall TAT, and the individual sub-step TATs (waiting room, pre-procedure, MRI scan, post-procedure durations) across hospitals because TATs were not normally distributed based on Shapiro–Wilk tests. We then used the Dunn post hoc test to verify pairwise comparisons and adjusted for multiple comparisons using the Benjamini–Hochberg procedure to control the false discovery rate. The Kruskal–Wallis and Pearson chi-square test compared continuous and categorical patient variables across hospitals (age and gender). We estimated determinants of overall TAT with an adjusted log-transformed multivariable linear regression model, stratified by MRI type with robust standard errors. We designated the hospital with the longest overall TAT as a reference for comparisons. We also designated the non-sedated brain MRI as a reference for comparison because it is the standard examination; we conceptualized that the sedated and limited brain MRIs are variations of the non-sedated examination. All hypothesis testing was two-sided, with statistical significance defined as P < 0.05.
Results
Overall, in our total sample, patient age varied across hospitals (P = 0.003) but not gender (P = 0.06). Patient characteristics across hospitals and by MRI type are detailed in Table 2. After stratifying by MRI type, children who underwent an MRI without sedation were older (median age: 12.9 years; IQR: 6.5 years; P = 0.85) than children who underwent an MRI with sedation (median age: 3.7 years; IQR: 4.3 years; P < 0.001) or a limited MRI scan (median age: 2.7 years; IQR: 9.3 years; P = 0.96). Patient gender did not vary across hospitals for children who underwent a non-sedated MRI (P = 0.59) versus an MRI with sedation (P = 0.11).
Overall turnaround time
Based on combined hospital data, the overall TAT and the imaging acquisition times each varied based on type of MRI examination (sedated versus non-sedated versus limited MRI) (medians presented in Tables 3 and 4). The average overall TAT was 185 min (standard deviation [SD]: 54 min) for a sedated MRI, 94 min (SD: 36 min) for a non-sedated MRI and 55 min (SD: 27 min) for a limited MRI. The average image acquisition time was 38 min (SD: 14 min) for a sedated MRI, 38 min (SD: 14 min) for a non-sedated MRI and 7 min (SD: 5 min) for a limited MRI (medians presented in Table 4). The range in times was also wide for overall TAT and image acquisition time, with TAT for a sedated brain MRI ranging from 158 min to 224 min, a non-sedated MRI from 70 min to 112 min and a limited MRI from 44 min to 70 min. We found similar wide ranges in image acquisition times by hospital.
Regarding the hospital pairwise comparisons, we found significant differences in each of the six comparisons in overall TAT for sedated examinations, meaning each hospital’s median overall TATs were significantly different (when compared to each of the other hospitals in the study). For non-sedated examinations, we found significant differences in overall TAT in three of the six pairwise comparisons, and among limited examinations there were significant differences in four of the six pairwise comparisons (Table 3).
Magnetic resonance imaging phases of care
For the sedated MRI pathway, the pre-procedure phase was the longest step at three of the four hospitals, ranging from 52 min to 93 min. Among the four hospitals, we also noted that the individual phases were significantly different (waiting room time, pre-procedure time, MRI acquisition time and post-procedure time, all P < 0.001) (Fig. 1). For instance, patient wait times ranged from 12 min at hospital C to 30 min at hospital A; pre-procedure duration varied from 52 min at hospital B to 93 min at hospital A; MRI acquisition time ranged from 27 min at hospital D to 46 min at hospital B. Post-procedure duration also varied from 43 min at hospital B to 64 min at hospital A.
Phase times for sedated vs. non-sedated vs. limited MRI at four pediatric academic hospitals. Graphs show mean phase durations in minutes. a Sedated MRI phases: waiting room, which consists of the time the child checks in (registration) to the start time of pre-procedure appointment; pre-procedure, which consists of the nurse pre-procedure evaluation, the technologist MRI safety screening and the pre-procedure anesthesiologist evaluation and consent; MRI, which consists of the MRI acquisition time; and post-procedure, which consists of transferring the child from the MRI scanner to the post-anesthesia care unit (PACU) and ends with PACU discharge. b Non-sedated MRI phases: waiting room, which consists of the time the child checks in (registration) to the time the technologist starts the MRI safety screening; pre-procedure, which consists of the technologist MRI safety screening; and MRI, which consists of the MRI acquisition time. c Limited MRI phases: waiting room, which consists of the time the child checks in (registration) to the time the technologist starts the MRI safety screening; pre-procedure, which consists of the technologist MRI safety screening; and MRI, which consists of the MRI acquisition time
For the non-sedated MRI pathway, the MRI scan phase was the longest step at each hospital, ranging from 28 min to 45 min (Fig. 1). We also noted that in non-sedated examinations, individual phase durations were significantly different across hospitals (waiting room time, pre-procedure time and MRI acquisition time; P < 0.001).
Regarding limited MRIs, the MRI scan phase among three of the hospitals was the shortest step because of the limited number of sequences included in these imaging protocols (Fig. 1). Additionally, individual phases were significantly different across hospitals for limited MRIs (waiting room time, pre-procedure time and MRI acquisition time; P < 0.001).
Predictors of overall turnaround time
Our multivariate regression model included the following predictors: patient age, patient gender, hospital and type of brain MRI (non-sedated, sedated, limited); it yielded an R-squared of 74%. The most significant predictor of a longer overall TAT was having a sedated MRI (coefficient = 0.71, 95% confidence interval [CI]: 0.66–0.75; P < 0.001). Thus, having a sedated MRI compared to a non-sedated MRI resulted in a 70% increase in overall TAT. Hospital site was also a significant predictor of overall TAT, with an MRI of the brain (regardless of MRI type) at hospital B resulting in a 20% decrease in overall TAT, compared to hospital A (reference hospital). Having an MRI of the brain at hospital C resulted in a 17% decrease in overall TAT, compared to hospital A, and having an MRI of the brain at hospital D resulted in a 39% decrease in overall TAT, compared to hospital A (Table 5). Patient age (P = 0.95) and patient gender (P = 0.80) were not significant predictors of overall TAT (Table 5).
Magnetic resonance imaging scan turnaround time and Relative Value Scale Update Committee practice expense times
The RUC lists the median practice expense time to perform an MRI of the brain without contrast agent, Current Procedural Terminology (CPT) 70,551, as 20 min [17]. Based on the four hospitals’ combined data, the median non-sedated MRI was 38 min and sedated MRI was 37 min. Both empirical scan times are approximately double what is used by the RUC’s practice expense subcommittee.
Discussion
This study expanded on a prior single-center report regarding MRI efficiency [12] by assessing the overall TAT for an outpatient non-contrast MRI brain examination among four pediatric academic institutions across the nation. Overall, we found significant differences by hospital (controlling for broad patient characteristics) in the overall TAT for an MRI of the brain. We also noted significant differences in individual phase times among hospitals. Specifically, for a sedated MRI, we found that children and families could expect the entire visit to take an average of 2.5–4 h, depending on hospital.
Across hospitals, sedation was a significant predictor of longer overall TAT. While we found younger ages to be related to sedation, ultimately patient age was not a significant predictor of overall TAT; rather, overall TAT was explained by the use of sedation in the examination. Conversely, we found that non-sedated examinations had a shorter overall TAT, with a door-in-door-out duration (time from arrival to discharge) of less than 1 h. These findings highlight the importance of sedation reduction strategies. Such reduction strategies, if successful, could result in significant efficiency gains for both hospitals and families.
A growing body of literature is focused on sedation reduction in pediatrics. This research is driven by safety concerns, heightened costs and appointment duration. Researchers have examined approaches to limiting the need for anesthesia and sedation for pediatric MRI, including distraction techniques, feed-and-swaddle methods, limited or rapid MRI acquisition techniques, the use of child life specialists and the use of mock MRI scanners [18,19,20,21,22]. However, to limit sedation, there is a need for studies that focus on scalable approaches across multiple institutions in the implementation science literature.
While we did not thoroughly explore workflow and operational factors, for sedated MRIs we noted that the pre-procedure workup was both the longest and most variable phase. Other research reports noted that pre-procedural workup (for minor procedures with anesthesia) was also the longest step because it is a complex process that spans multiple personnel types, including anesthesiologists, MRI technologists, nurses and child life specialists [23, 24]. Considerable variability also exists with regard to how the pre-procedure workups are completed. For instance, in some radiology departments, the anesthesiologists perform the entire pre-procedure evaluation, whereas in other departments this process is conducted by nurse anesthetists or facilitated by nurse practitioners [25]. The variability of providers, coupled with high-complexity patients, can result in workflow inefficiencies, but also highlights opportunities for efficiency gains.
Furthermore, despite similar MRI brain protocols, we noted significant differences in overall TAT across the four hospitals. This reflects endogenous organizational factors, such as workflows, the number of personnel and the experience of the clinical team. While these factors were outside the scope of this cross-sectional study, some points are worth noting. For instance, we observed that two hospitals with shorter overall TAT (on sedated MRI examinations) used status boards with color-coded tracking to identify where the child is along the MRI workflow. The status or tracking boards facilitate communication between staff and providers. Alternatively, one of the hospitals with longer overall TAT relied on the medical record for communication (which needed manual refreshing) as well as phone calls. Future implementation science research should explore these operational factors in-depth and their impact on MRI workflow and efficiency across institutions.
We also examined MRI acquisition time (the duration the MRI technologist spends scanning, positioning the child and instructing the child) and found that practice expense RVUs underestimate the empirical times from our study. This finding has policy implications about how medical care, and specifically pediatric imaging examinations, are valued. CMS and the resource-based relative value scale (RBRVS) use a top-down costing approach to estimate the cost of medical care and procedures [7,8,9]. Current time estimates for RVU valuations are conducted through physician surveys; however, a major limitation with these surveys is a low response rate, such as 2.2% [4, 26]. CMS previously expressed concerns that the procedural time estimates, a central component to RVU valuations, might be biased [4]. An alternative and more accurate way of assessing time estimates would be from empirical data. However, these data can be difficult or impractical to obtain — even in our current study we conducted manual data collection for two of the four hospitals.
Previous studies, mostly focusing on surgical specialties, compared procedure duration from empirical time stamps to the CMS duration values and concluded that the RUC estimates used by CMS were biased [27,28,29,30,31]. A recent study published in the New England Journal of Medicine analyzed the RUC time valuations of 293 surgical procedures and compared these estimates to empirical time estimates located in the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database [28]. As in our study, the authors found substantial differentials between intraoperative times used by RUC and the empirical time estimates [28].
Additionally, the estimates used by the RUC are based on examinations in the adult population instead of the pediatric population. Because the RUC estimates are primarily used to inform the Medicare payment and fee schedule, the vignettes use the adult patient as the base case in their “standard patient narrative.” It is possible that the adult examinations do not fully translate to pediatrics. Pediatric imaging requires an intensive, alternative approach that can be less efficient when compared to adult imaging. Our study demonstrates the need for more pediatric-centric benchmarking for accurate RVU valuation. These advanced imaging time durations can also be used for operational benchmarking, where institutions can measure their own efficiency against a national or regional metric, similar to the American College of Radiology’s radiation index registry that provides radiation dose reference levels for common pediatric CTs [32]. More pediatric studies examining the accuracy of the RUC turnaround time estimates are needed to validate our findings on a national level and to provide national benchmarking of TAT.
Our study had several limitations, the first of which concerns with sample size. While our study included a large number of subjects, it is possible that if we included more pediatric institutions and a larger time span of data that our time estimates would be different from those reported in the current study. We based our study on a sample size that was calculated from previous literature [12, 16] because the data requirements for reviewing more examinations across the four institutions were impractical and would have required extensive manual EMR review. Second, we were not able to collect data on the specialized MRI protocols (e.g., seizure, brain tumor, etc.), because these data were not easily accessible in the EMR. It is possible that the TATs, specifically the scan acquisition times, would differ by MRI protocol by institution. However, the aim of the study was to derive combined institutional turnaround times of brain MRIs, regardless of the specific protocol. Third, we only included academic pediatric hospitals and thus our results might not be generalizable to private practice imaging centers.
Conclusion
This study builds upon single-center studies of MRI efficiency and throughput by defining cross-institutional metrics for benchmarking time estimates. We found considerable differences in the overall TAT across four pediatric academic institutions. Overall, the significant predictors of turnaround times were hospital site and MRI pathway (non-sedated versus sedated versus limited MRI). In our sample, we also found that patient characteristics such as age and gender were not significant predictors of overall MRI turnaround time. We consistently found (at each hospital) that the turnaround time in performing sedated MRI of the brain was twice that of a non-sedated MRI of the brain. Finally, regarding the MRI scan duration component, we found that the median MRI scan times at each of the institutions were double what is used in the practice expense RVU calculation by the RUC. Future studies should focus on MRI costs across institutions to inform value-based payment reforms.
References
Buller M, Karis J (2017) Introduction of a dedicated emergency department MR imaging scanner at the Barrow Neurological Institute. AJNR Am J Neuroradiol 38:1480–1485
Chong ST, Robinson JD, Davis MA et al (2019) Emergency radiology: current challenges and preparing for continued growth. J Am Coll Radiol 16:1447–1455
Mason KP (2010) Sedation trends in the 21st century: the transition to dexmedetomidine for radiological imaging studies. Pediatr Anesth 20:265–272
Zuckerman S, Merrell K, Berenson R et al (2016) Collecting empirical physician time data: piloting an approach for validating work relative value units. Urban Institute, Washington, DC
Smith SD, Gerstle RS, Andreae MC et al (2008) Application of the resource-based relative value scale system to pediatrics. Pediatrics 122:1395–1400
Gerstle RS, Molteni RA, Andreae MC et al (2014) Application of the resource-based relative value scale system to pediatrics. Pediatrics 133:1158–1162
Lam DL, Medverd JR (2013) How radiologists get paid: resource-based relative value scale and the revenue cycle. AJR Am J Roentgenol 201:947–958
Petrey WB, Allen B Jr, Thorwarth WT Jr (2009) Radiology coding, reimbursement, and economics: a practical playbook for housestaff. J Am Coll Radiol 6:643–648
Weiner S, Tu R, Javan R, Taheri M (2018) Health care economics: a study guide for neuroradiology fellows, part 2. AJNR Am J Neuroradiol 39:10–17
Smereka P, Weng J, Block KT, Chandarana H (2022) Factors affecting MRI scanner efficiency in an academic center. Abdom Radiol 47:3909–3915
Streit U, Uhlig J, Lotz J et al (2021) Analysis of core processes of the MRI workflow for improved capacity utilization. Eur J Radiol 138:109648
Vanderby SA, Babyn PS, Carter MW et al (2010) Effect of anesthesia and sedation on pediatric MR imaging patient flow. Radiology 256:229–237
Olmsted MG, Powell, R, Murphy J et al (2020) Methodology: U.S. News & World Report best children’s hospitals 2020–21. RTI International. https://www.rti.org/publication/methodology-13/fulltext.pdf. Accessed 2 Nov 2022
Chang G, Doshi A, Chandarana H, Recht M (2021) Impact of COVID-19 workflow changes on patient throughput at outpatient imaging centers. Acad Radiol 28:297–306
Mezrich JL, Weinreb JC (2022) Financial and medicolegal implications of focused/fast abdominopelvic MRI exams. Abdom Radiol 47:471–474
Patel DM, Tubbs RS, Pate G et al (2014) Fast-sequence MRI studies for surveillance imaging in pediatric hydrocephalus. J Neurosurg Pediatr 13:440–447
National Archives (2013) Federal Register 78(237):73993–74682
Carter AJ, Greer M-LC, Gray SE, Ware RS (2010) Mock MRI: reducing the need for anaesthesia in children. Pediatr Radiol 40:1368–1374
Harrington SG, Jaimes C, Weagle KM et al (2022) Strategies to perform magnetic resonance imaging in infants and young children without sedation. Pediatr Radiol 52:374–381
Jaimes C, Kirsch JE, Gee MS (2018) Fast, free-breathing and motion-minimized techniques for pediatric body magnetic resonance imaging. Pediatr Radiol 48:1197–1208
Rothman S, Gonen A, Vodonos A et al (2016) Does preparation of children before MRI reduce the need for anesthesia? Prospective randomized control trial. Pediatr Radiol 46:1599–1605
Windram J, Grosse-Wortmann L, Shariat M et al (2012) Cardiovascular MRI without sedation or general anesthesia using a feed-and-sleep technique in neonates and infants. Pediatr Radiol 42:183–187
Abouleish AE, Dexter F, Whitten CW et al (2004) Quantifying net staffing costs due to longer-than-average surgical case durations. Anesthesiology 100:403–412
Schuster M, Standl T, Wagner JA et al (2004) Effect of different cost drivers on cost per anesthesia minute in different anesthesia subspecialties. Anesthesiology 101:1435–1443
Wittkugel E, Varughese A (2015) Development of a nurse-assisted preanesthesia evaluation program for pediatric outpatient anesthesia. Pediatr Anesth 25:719–726
Buntin MB, Zuckerman S, Berenson R et al (2008) Volume growth in medicine: an investigation of ten physicians’ services. RAND Health. Urban Institute, Washington, DC
Chakiryan NH, Jiang DD, Gillis KA et al (2020) RUC operative time estimates are inaccurate, resulting in decreased work RVU assignments for longer urologic procedures. Urology 142:94–98
Chan DC, Huynh J, Studdert DM (2019) Accuracy of valuations of surgical procedures in the Medicare fee schedule. N Engl J Med 380:1546–1554
Sebro R (2021) Leveraging the electronic health record to evaluate the validity of the current RVU system for radiologists. Clin Imaging 78:286–292
Shah DR, Bold RJ, Yang AD et al (2014) Relative value units poorly correlate with measures of surgical effort and complexity. J Surg Res 190:465–470
Sodhi N, Piuzzi NS, Khlopas A et al (2018) Are we appropriately compensated by relative value units for primary vs revision total hip arthroplasty? J Arthroplasty 33:340–344
Kanal KM, Butler PF, Chatfield MB et al (2022) US diagnostic reference levels and achievable doses for 10 pediatric CT examinations. Radiology 302:164–174
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hayatghaibi, S.E., Cazaban, C.G., Chan, S.S. et al. Turnaround time and efficiency of pediatric outpatient brain magnetic resonance imaging: a multi-institutional cross-sectional study. Pediatr Radiol 53, 1144–1152 (2023). https://doi.org/10.1007/s00247-022-05563-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-022-05563-9