Abstract
Background
Susceptibility-weighted imaging (SWI) is highly sensitive for intracranial hemorrhagic and mineralized lesions but is associated with long scan times. Wave controlled aliasing in parallel imaging (Wave-CAIPI) enables greater acceleration factors and might facilitate broader application of SWI, especially in motion-prone populations.
Objective
To compare highly accelerated Wave-CAIPI SWI to standard SWI in the non-sedated pediatric outpatient setting, with respect to the following variables: estimated scan time, image noise, artifacts, visualization of normal anatomy and visualization of pathology.
Materials and methods
Twenty-eight children (11 girls, 17 boys; mean age ± standard deviation [SD] = 128.3±62 months) underwent 3-tesla (T) brain MRI, including standard three-dimensional (3-D) SWI sequence followed by a highly accelerated Wave-CAIPI SWI sequence for each subject. We rated all studies using a predefined 5-point scale and used the Wilcoxon signed rank test to assess the difference for each variable between sequences.
Results
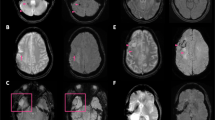
Wave-CAIPI SWI provided a 78% and 67% reduction in estimated scan time using the 32- and 20-channel coils, respectively, corresponding to estimated scan time reductions of 3.5 min and 3 min, respectively. All 28 children were imaged without anesthesia. Inter-reader agreement ranged from fair to substantial (k=0.67 for evaluation of pathology, 0.55 for anatomical contrast, 0.3 for central noise, and 0.71 for artifacts). Image noise was rated higher in the central brain with wave SWI (P<0.01), but not in the peripheral brain. There was no significant difference in the visualization of normal anatomical structures and visualization of pathology between the standard and wave SWI sequences (P=0.77 and P=0.79, respectively).
Conclusion
Highly accelerated Wave-CAIPI SWI of the brain can provide similar image quality to standard SWI, with estimated scan time reduction of 3–3.5 min depending on the radiofrequency coil used, with fewer motion artifacts, at a cost of mild but perceptibly increased noise in the central brain.




Similar content being viewed by others
References
Haacke EM, Mittal S, Wu Z et al (2009) Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol 30:19–30
Sodickson DK, Manning WJ (1997) Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magn Reson Med 38:591–603
Shams S, Martola J, Cavallin L et al (2015) SWI or T2*: which MRI sequence to use in the detection of cerebral microbleeds? The Karolinska imaging dementia study. AJNR Am J Neuroradiol 36:1089–1095
Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P (1999) SENSE: sensitivity encoding for fast MRI. Magn Reson Med 42:952–962
Griswold MA, Jakob PM, Heidemann RM et al (2002) Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 47:1202–1210
Bilgic B, Gagoski BA, Cauley SF et al (2015) Wave-CAIPI for highly accelerated 3D imaging. Magn Reson Med 73:2152–2162
Moriguchi H, Duerk JL (2006) Bunched phase encoding (BPE): a new fast data acquisition method in MRI. Magn Reson Med 55:633–648
Haacke E, Tang J, Neelavalli J, Cheng Y (2010) Susceptibility mapping as a means to visualize veins and quantify oxygen saturation. J Magn Reson Imaging 32:663–676
Cauley SF, Setsompop K, Bilgic B et al (2017) Autocalibrated wave-CAIPI reconstruction; joint optimization of k-space trajectory and parallel imaging reconstruction. Magn Reson Med 78:1093–1099
Ahn S, Park SH, Lee KH (2013) How to demonstrate similarity by using noninferiority and equivalence statistical testing in radiology research. Radiology 267:328–338
Goncalves Filho ALM, Conklin J, Longo MGF et al (2020) Accelerated post-contrast wave-CAIPI T1 SPACE achieves equivalent diagnostic performance compared with standard T1 SPACE for the detection of brain metastases in clinical 3T MRI. Front Neurol 11:587327
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Lakens D, Scheel AM, Isager PM (2018) Equivalence testing for psychological research: a tutorial. Adv Methods Pract Psychol Sci 1:259–269
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum, New York
Feinstein AR, Cicchetti DV (1990) High agreement but low kappa: I. the problems of two paradoxes. J Clin Epidemiol 43:543–549
Conklin J, Longo MGF, Cauley SF et al (2019) Validation of highly accelerated wave-CAIPI SWI compared with conventional SWI and T2*-weighted gradient recalled-echo for routine clinical brain MRI at 3T. AJNR Am J Neuroradiol 40:2073–2080
Balza R, Jaimes C, Risacher S et al (2019) Impact of a fast free-breathing 3-T abdominal MRI protocol on improving scan time and image quality for pediatric patients with tuberous sclerosis complex. Pediatr Radiol 49:1788–1797
Dong SZ, Zhu M, Bulas D (2019) Techniques for minimizing sedation in pediatric MRI. J Magn Reson Imaging 50:1047–1054
Ahmad R, Hu HH, Krishnamurthy R et al (2018) Reducing sedation for pediatric body MRI using accelerated and abbreviated imaging protocols. Pediatr Radiol 48:37–49
Sheridan DC, Newgard CD, Selden NR et al (2017) QuickBrain MRI for the detection of acute pediatric traumatic brain injury. J Neurosurg Pediatr 19:259–264
Christy A, Murchison C, Wilson JL (2018) Quick brain magnetic resonance imaging with diffusion-weighted imaging as a first imaging modality in pediatric stroke. Pediatr Neurol 78:55–60
Prakkamakul S, Witzel T, Huang S et al (2016) Ultrafast brain MRI: clinical deployment and comparison to conventional brain MRI at 3T. J Neuroimaging 26:503–510
Nandigam RN, Viswanathan A, Delgado P et al (2009) MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol 30:338–343
Tabari A, Conklin J, Figueiro Longo MG et al (2021) Comparison of ultrafast wave-controlled aliasing in parallel imaging (CAIPI) magnetization-prepared rapid acquisition gradient echo (MP-RAGE) and standard MP-RAGE in non-sedated children: initial clinical experience. Pediatr Radiol 51:2009–2017
Jaimes C, Gee MS (2016) Strategies to minimize sedation in pediatric body magnetic resonance imaging. Pediatr Radiol 46:916–927
Jaimes C, Murcia DJ, Miguel K et al (2018) Identification of quality improvement areas in pediatric MRI from analysis of patient safety reports. Pediatr Radiol 48:66–73
Metzner J, Domino KB (2010) Risks of anesthesia or sedation outside the operating room: the role of the anesthesia care provider. Curr Opin Anaesthesiol 23:523–531
Vanderby SA, Babyn PS, Carter MW et al (2010) Effect of anesthesia and sedation on pediatric MR imaging patient flow. Radiology 256:229–237
Acknowledgments
John Conklin and Azadeh Tabari contributed equally to this publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Conklin, J., Tabari, A., Longo, M.G.F. et al. Evaluation of highly accelerated wave controlled aliasing in parallel imaging (Wave-CAIPI) susceptibility-weighted imaging in the non-sedated pediatric setting: a pilot study. Pediatr Radiol 52, 1115–1124 (2022). https://doi.org/10.1007/s00247-021-05273-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-021-05273-8