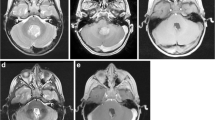
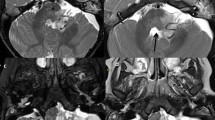
Abstract
MRI is the ideal modality for imaging intracranial tumours. Intraoperative MRI (ioMRI) makes it possible to obtain scans during a neurosurgical operation that can aid complete macroscopic tumour resection — a major prognostic factor in the majority of brain tumours in children. Intra-operative MRI can also help limit damage to normal brain tissue. It therefore has the potential to improve the survival of children with brain tumours and to minimise morbidity, including neurological deficits. The use of ioMRI is also likely to reduce the need for second look surgery, and may reduce the need for chemotherapy and radiotherapy. High-field MRI systems provide better anatomical information and also enable effective utilisation of advanced MRI techniques such as perfusion imaging, diffusion tensor imaging, and magnetic resonance spectroscopy. However, high-field ioMRI facilities require substantial capital investment, and careful planning is required for optimal benefit. Safe ioMRI requires meticulous attention to detail and rigorous application of magnetic field safety precautions. Interpretation of ioMRI can be challenging and requires experience and understanding of artefacts that are common in the intra-operative setting.










Similar content being viewed by others
References
Albright AL, Wisoff JH, Zeltzer PM et al (1996) Effects of medulloblastoma resections on outcome in children: a report from the Children’s Cancer Group. Neurosurgery 38:265–271
Wisoff JH, Boyett JM, Berger MS et al (1998) Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children’s Cancer Group trial no. CCG-945. J Neurosurg Paediatr 89:52–59
Rodriguez D, Cheung MC, Housri N et al (2009) Outcomes of malignant CNS ependymomas: and examination of 2408 cases through the Surveillance, Epidemiology and End Results (SEER) database (1973–2005). J Surg Res 156:340–355
Buchfelder M, Ganslandt O, Fahlbusch R et al (2000) Intraoperative magnetic resonance imaging in epilepsy surgery. J Magn Reson Imaging 12:547–555
Buchfelder M, Fahlbusch R, Ganslandt O et al (2002) Use of intraoperative magnetic resonance imaging in tailored temporal lobe surgeries for epilepsy. Epilepsia 43:864–873
Pamir MN, Ozduman K, Dincer A et al (2010) First intraoperative, shared-resource, ultrahigh-field 3-Tesla magnetic resonance imaging system and its application in low grade glioma resection. J Neurosurg 112:57–69
Nimsky C, Ganslandt O, Von Keller B et al (2004) Intraoperative high-field-strength MR imaging: implementation and experience in 200 patients. Radiology 233:67–78
Knauth M, Wirtz CM, Tronnier VM et al (1999) Intraoperative MR imaging increases the extent of tumor resection in patients with high-grade gliomas. AJNR 20:1642–1646
Wirtz CR, Albert FK, Schwaderer M et al (2000) The benefit of neuronavigation for neurosurgery analyzed by its impact on glioblastoma surgery. Neurol Res 22:354–360
Nimsky C, von Keller B, Schlaffer S et al (2008) Updating navigation with intraoperative image data. Top Magn Res Imaging 19:197–204
Okada T, Mikuni N, Miki Y et al (2006) Corticospinal tract localization: integration of diffusion-tensor tractography at 3-T MR imaging with intraoperative white matter stimulation mapping—preliminary results. Radiology 240:849–857
Nimsky C, Ganslandt O, Hastreiter P et al (2005) Intraoperative diffusion-tensor MR imaging: shifting of white matter tracts during neurosurgical procedures—initial experience. Radiology 234:218–225
Kremer P, Tronnier V, Steiner HH et al (2006) Intraoperative MRI for interventional neurosurgical procedures and tumor resection control in children. Childs Nerv Syst 22:674–678
Levy R, Cox RG, Hader WJ et al (2009) Application of intraoperative high-field magnetic resonance imaging in pediatric neurosurgery. J Neurosurg Pediatr 4:467–474
Avula S, Mallucci CL, Pizer B et al (2011) 3-Tesla intraoperative MRI in the management of paediatric cranial tumours—initial experience. Pediatr Radiol. doi:10.1007/s00247-011-2261-6
Ivanov M, Wilkins S, Poeata I et al (2010) Intraoperative ultrasound in neurosurgery—a practical guide. Br J Neurosurg 24:510–517
Arbel T, Morandi X, Comeau RM et al (2004) Automatic non-linear MRI-ultrasound registration for the correction of intra-operative brain deformations. Comput Aided Surg 9:123–136
Knauth M, Aras N, Wirtz CR et al (1999) Surgically induced intracranial contrast enhancement: potential source of diagnostic error in intraoperative MR imaging. AJNR 20:1547–1553
Widjaja E, Connolly DJ, Gatscher S et al (2005) Spurious leptomeningeal enhancement on immediate post-operative MRI for paediatric brain tumours. Pediatr Radiol 35:334–338
Loree J, Mehta V, Bhargarva R (2010) Cranial magnetic resonance imaging findings of leptomeningeal contrast enhancement after pediatric posterior fossa tumor resection and its significance. J Neurosurg Pediatr 6:87–91
Weber MA, Zoubaa S, Schlieter M et al (2006) Diagnostic performance of spectroscopic and perfusion MRI for distinction of brain tumors. Neurology 66:1899–1906
Ulmer S, Hartswigen G, Riedel C et al (2010) Intraoperative dynamic susceptibility contrast MRI (iDSC-MRI) is as reliable as preoperatively acquired perfusion mapping. NeuroImage 49:2158–2162
Sadowski EA, Bennett LK, Chan MR et al (2007) Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 243:148–157
Deibler AR, Pollock JM, Kraft RA et al (2008) Arterial spin labelling in routine clinical practice: technique and artifacts. AJNR 29:1228–1234
Jarnum H, Steffensen EG, Knutsson L et al (2010) Perfusion MRI of brain tumours: a comparative study of pseudo-continuous arterial spin labelling and dynamic susceptibility contrast imaging. Neuroradiology 52:307–317
Warmuth C, Gunther M, Zimmer C (2003) Quantification of blood flow in brain tumors: comparison of arterial spin labeling and dynamic susceptibility-weighted contrast-enhanced MR imaging. Radiology 228:523–532
Weber MA, Gunther M, Lichy MP et al (2003) Comparison of arterial spin-labeling techniques and dynamic susceptibility-weighted contrast-enhanced MRI in perfusion imaging of normal brain tissue. Investig Radiol 38:712–718
Wolf RL, Wang J, Wang S et al (2005) Grading of CNS neoplasms using continuous arterial spin labeled perfusion MR imaging at 3 Tesla. J Magn Reson Imaging 22:475–482
Weber MA, Thilmann C, Lichy MP et al (2004) Assessment of irradiated brain metastases by means of arterial spin-labeling and dynamic susceptibility-weighted contrast-enhanced perfusion MRI: Initial results. Investig Radiol 39:277–287
Waldman AD, Jackson A, Price SJ et al (2009) Quantitative imaging biomarkers in neuro-oncology. Nat Rev Clin Oncol 6:445–454
O’Connor JP, Jackson A, Asselin MC et al (2008) Quantitative imaging biomarkers in the clinical development of targeted therapeutics: current and future perspectives. Lancet Oncol 9:766–776
Parkes LM (2005) Quantification of cerebral perfusion using arterial spin labeling: two-compartment models. J Magn Reson Imaging 22:732–736
Liu Y, Fedorov A, Kikinis R et al (2010) Non-rigid registration for brain MRI: faster and cheaper. Int J Functional Informatics Personalised Med 3:1756–2112
Acknowledgements
The Barclay Foundation; The Alder Hey Imagine Appeal; Philips Healthcare, Best, The Netherlands; NORAS MRI Products GmbH, Hochberg, Germany; BrainLAB, Feldkirchen, Germany
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abernethy, L.J., Avula, S., Hughes, G.M. et al. Intra-operative 3-T MRI for paediatric brain tumours: challenges and perspectives. Pediatr Radiol 42, 147–157 (2012). https://doi.org/10.1007/s00247-011-2280-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-011-2280-3