Abstract
Interventional radiology in children involves nearly every aspect of infectious disease. Diagnosis, treatment, prophylaxis and disease transmission in infectious disease are a daily part of pediatric interventional radiology practice. This article will discuss each of these aspects of infection with respect to interventional radiology.
Similar content being viewed by others
Introduction
Common infections in children include pneumonia (bacterial or viral), abscesses throughout the body, meningitis, and iatrogenic infections, such as central venous catheter-related bacteremia and sepsis. Interventional radiology (IR) has a major role in the diagnosis and treatment of pediatric infectious diseases. Unfortunately, IR is in a position to cause infections in children due to procedural and postprocedural complications, and IR is a risk factor for spreading infection between patients or to health care workers.
Diagnosis and treatment
Aspiration and drainage
Image-guided techniques with aspiration or catheter placement have been used to treat abscesses in almost every part of the body. In the abdomen, appendicitis is the most common etiology of an abscess, and it may present before or after surgery [1–8]. Other etiologies for abdominal collections include cerebrospinal fluid pseudocysts (Fig. 1), post-traumatic collections, pancreatic fluid collections, alcalculous cholecystitis, necrotizing enterocolitis, inflammatory bowel disease, ascites, renal etiologies such as pyonephrosis, or abscess [7–9].
In the thorax, IR drainage and aspiration procedures are used to diagnose or treat empyema and lung abscesses [10–16] and possible pulmonary infections (especially aspergillus) [17–19]. Soft-tissue infections, septic joint effusions and osteomyelitis are also sites for IR drainage and aspiration [7]. We are often asked to perform image-guided lumbar punctures for suspected meningitis.
Drainage may be performed if a fluid collection is present and if any of the following are pertinent [21, 22]:
-
1.
There is a suspicion of infection or fistula.
-
2.
There is a need for fluid characterization.
-
3.
The size causes symptoms warranting drainage.
-
4.
Drainage can temporize symptoms until definite surgery.
-
5.
Drainage is an adjunctive to another procedure.
Contraindications to drainage are all relative and include [22]:
-
1.
Coagulopathy.
-
2.
Lack of a safe pathway.
-
3.
Severe cardiopulmonary compromise or hemodynamic instability.
-
4.
Inability of patient to cooperate.
Regardless of where the infection is, the basic goal is to use image guidance to place a needle into the area. If only aspiration is desired, the entire procedure may be done through the needle. If a larger drainage device is required, or if the patient will have continuous drainage, the Seldinger technique is used: A wire is advanced, the tract is dilated and a catheter is placed [7, 8]. The most commonly placed catheters are pigtail type with sizes ranging from 5–12 Fr. Alternatively, the catheter may be primarily placed with a trocar technique [23].
Imaging is predominately done with ultrasound [7, 8]. Advantages include real-time imaging, multiplanar capability and the avoidance of radiation. Since patients may vary from <1 kg to more than 200 kg, appropriate equipment must be available and the radiologist must be familiar with multiple transducers. In addition to initial needle placement, ultrasound can often be used for the entire procedure, including wire placement, tract dilatation and catheter deployment. Fluoroscopy may also be used for these steps. If there is interposed bone or air between the access site and the collection, CT or fluoroscopy may be substituted if appropriate [7, 8].
Since patient cooperation for the procedure is paramount for safety, almost all patients will benefit from sedation or anesthesia with appropriate monitoring of vital signs [24].
Abdominal/pelvic abscesses
Access to abdominal abscesses and collections can be transabdominal, transgluteal or transrectal, and ultrasound guidance can be either transabdominal or transrectal (Figs. 2 and 3) [1–8, 25]. When using transrectal access, a trocar technique may be used as it protects the patient and radiologist from the sharp needle tip [3, 7, 8].
In all cases of suspected infection, a specimen should be sent to the laboratory for appropriate tests depending on the clinical question. Clinical success is reported in 81–100% of patients with most catheters removed within 3 days [7, 8, 22]. In up to 11% of patients, complications can occur, including catheter migration, hemorrhage, bacteremia or sepsis, and bowel or pleura injury. Major complications occur in less than 5% [7, 8, 22].
Empyema
Empyema can complicate pneumonia in up to 0.6% [26]. Streptococcus/pneumococcus is the most common etiology, although Staphylococcal pneumonia, mixed bacteria and other bacteria have a higher associated incidence of empyema (7/100,000 versus 10.3/100,000) [26]. Mixed bacteria and other bacteria may also cause empyema. Image-guided drainage of empyema has been described during the last decade and is a valuable alternative to video-assisted thoracoscopic surgery (VATS) (Fig. 4). Catheters are placed similarly to abdominal drains, with attention placed on putting the catheter in a posterior position and accessing over a rib to avoid injuring the intercostal artery or vein. Typical catheter size is 8–12 Fr and the catheters are placed to −20 cm H2O suction. Ultrasound prior to the procedure can demonstrate whether a pleural collection is present and it allows for the identification of septations. When septations are present, the intrapleural instillation of fibrinolytic agents will be required to ensure complete drainage. Fibrinolytic agents break fibrous strands, and clear lymphatic pores for fluid resorption [27]. Tissue plasminogen activator (rt-PA), urokinase and streptokinase have all been described, although streptokinase is rarely used due to concerns of anaphylaxis [10, 11, 28, 29]. These agents are injected directly into the pleural space via the catheter, which is then clamped for approximately 1 h prior to replacing the suction tube. Described dosing protocols for rt-PA are 2 mg in 20 ml normal saline twice a day or 0.1 mg/kg up to 4 mg in 20 ml three times a day [10, 11]. For urokinase, described dosing includes 3100 u/kg/day (administered as 1000 u/ml and a maximum dose of 100 ml) and 56,000 u/m2 (also administered as 1000 u/ml) [28, 29]. The pleural space is cleared of fluid and septations within the first few days, and drainage is continued until the tube output decreases. The maximum drainage before tube removal should be considered variable, but 20 ml/day or less is likely appropriate [10, 26]. Image-guided small-bore tube placement with fibrinolytic agents has a success rate of 84.5–99%, which is comparable to VATS [10, 11, 27, 30, 31]. Both are superior to tube placement alone. One metric often used to compare techniques is the overall patient length of stay (LOS). The three published randomized control studies show that this is similar between tube/fibrinolytics and VATS, has a mean of approximately 10 days, and the decreased invasiveness and lower cost of the IR procedure supports its use as a first-line therapy [30, 32–35]. Complications occur in less than 3% of patients, including pleural bleeding and bronchopleural fistula formation (though fistulas are thought to be more likely secondary to the actual disease process), while VATS complications may be seen in up to 13.8% of patients [10, 11, 26, 27, 30, 31, 36].
An 18-month-old with fever and cough. a The radiograph shows a right-side pneumonia with effusion. b Ultrasound guidance is used to place a needle into the septated pleural space for drainage. c After 3 doses of tissue plasminogen activator (rt-PA), the right pleural space is free of fluid with some residual pneumonia
Lung abscesses can be directly treated with catheter drainage as well (Fig. 5) [12–15]. While pulmonary abscesses usually respond to long-term antibiotic therapy, drainage may be indicated in very ill children and can improve symptoms more rapidly.
Other locations of abscesses can be successfully drained but may require specially sized catheters and careful technique. For small abscesses in the head and neck, smaller catheters should be used (French size and/or pigtail diameter) [8]. Conversely, pancreatic collections may require larger tubes. Percutaneous nephrostomies may be difficult in younger children because of the plasticity of the kidney, which makes catheter deployment more difficult [37]. If renal fungal balls are present, amphotericin or streptokinase instillation may be used [38, 39].
Lung biopsy/aspiration
A primary concern in immunocompromised patients with pulmonary lesions is aspergillus. Aspiration/biopsy may be indicated if the diagnosis cannot be made by other means; it has success rates of up to 97% [17–19]. However, there is also a fairly high risk of pulmonary hemorrhage (up to 46%). The benefits and risks of the procedure should be considered and discussed with the patient and family [19]. Other infectious entities can be sampled in the same manner.
Lumbar puncture
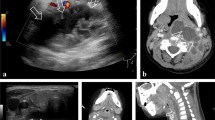
Most patients referred for possible infection are infants and young children with sepsis and suspected meningitis. We are increasingly being requested to perform these procedures on children. If the initial attempt is unsuccessful, ultrasound has shown that there is typically either an epidural hematoma compressing the cerebrospinal fluid out of the lower thecal sac or a hemorrhage within the thecal sac (Fig. 6) [20]. Attempting another lumbar puncture with these findings is usually unsuccessful. Daily ultrasound can be performed and attempts withheld until adequate fluid is identified in the thecal sac. Sedation can improve the chance of success, and patient positioning in a semi-upright position may allow gravity to fill the lower thecal sac to a greater degree. Ultrasound can be used to directly guide the needle into the CSF or it can confirm position after needle placement. When fluid is identified, this technique is nearly 100% successful [20].
This infant with fever and sepsis had attempts at lumbar puncture in the emergency department that were unsuccessful. a US of the spine shows epidural fluid (*) compressing the thecal sac. b Two days later, the epidural fluid has resolved and US guidance allows for a successful needle placement (arrow)
Procedurally related infections and prophylaxis
Central venous catheters
Any device placed percutaneously into the body has a risk of introducing infection. In central venous catheters (CVC), the incidence of infection varies depending on [40]:
-
1.
Type of catheter (peripheral insertion, central insertion, tunneled, totally implanted).
-
2.
Frequency of catheter manipulations.
-
3.
Patient-related factors.
The definition of a catheter-related bloodstream infection (CR-BSI) is a positive blood culture from a peripheral vein, clinical manifestations of infection and no apparent source except the catheter [41]. Overall infection rates are 2.3–7.8/1,000 catheter days, although this may be an overestimation as infection from another unknown source may be attributed to the catheter [41–45]. ICU LOS may increase 14.6 days and total hospital LOS 21.1 d [43]. Attributed patient mortality is 12–25% for each line infection [41]. This increases patient cost with each CVC-related infection having an estimated cost of $35,000–$56,000 for ICU patients and $25,000 for all hospital patients [40, 41, 43].
Coagulase negative staphylococcus is the most common cause and comprises 37–60% of line infections [40, 41, 46]. Enterococcus is responsible in 13.5% of infected patients (with 25.9% being resistant to vancomycin), gram-negative rods in 14% and candida in 8% [40, 41]. Line infection typically is from the migration of skin organisms along the catheter surface to the catheter tip for CVCs and peripherally inserted central catheters (PICCs), while hub contamination is the cause in long-term tunneled and totally implanted catheters [40, 41]. Therefore, the fewest number of hubs possible should be used, and tunneled catheters (accessed often) or ports (accessed only intermittently) are recommended for long-term care [40, 47].
Several factors have been shown to affect the incidence of infection [40, 41]. Infection may be more common in CVCs placed via the internal jugular vein than in those placed via a subclavian vein or femoral vein [40, 41, 48]. However, the subclavian vein also has a higher incidence of CVC-associated thrombosis [49]. Therefore, the site of access needs to be considered with respect to the patient’s needs and risks.
Insertion should be with maximum barrier technique, including cap, mask, gown, gloves and a large sterile sheet [40, 45]. Chlorhexidene 2% is the recommend skin prep for patients older than 2 months, with povidone-iodine used in younger patients or those weighing less than 1,000 gm [40].
Catheter coatings with chlorhexidine/silver sulfadiazide or minocycline/rifampin lower the incidence of bloodstream infections (BSIs), but ionic silver impregnated cuffs have not proven effective [40, 41]. Suture-less fixation devices have been shown to reduce infection rates [40, 50].
Antibiotic catheter lock prophylaxis has also been tried [41, 51, 52]. Ethanol (70%) has been used and shown effect in small series of patients [51, 52]. Vancomycin with or without ciprofloxacin may lower the rate of CR-BSI significantly, but this may induce vancomycin resistance and is not routinely recommended [41].
Catheters should not be routinely replaced [41]. Catheters should not be removed on the basis of fever alone, as infection is only present in 15–41% of cases [46]. In addition, no potentially infected catheter should be replaced through the same access over a guidewire.
Due to the increased morbidity, mortality and costs associated with CVC-related BSIs, programs and interventions have occurred in an attempt to lower their incidence. This includes filling out an insertion bundle, a compliance checklist including tasks such as hand-washing, site preparation and proper draping [43], the use of appropriate disinfectant, the placement of a chlorhexidine-impregnated disc at the insertion site, and feedback on results [41, 53]. In one study, this decreased infection rates from 7.8/1,000 catheter days to 2.3/1,000 catheter days with an overall bundle compliance of 94% [43]. In a study performed in a pediatric ICU, there was a similar decrease in infections from 5.4 to 3.1/1,000 catheter days [44]; however, they determined that more improvement was due to compliance in daily care than during insertion [44].
Procedural antibiotic prophylaxis
There have been a few articles detailing the use of prophylactic antibiotics in IR patients, the latest update by Ryan et al. [47]. The value of prophylactic antibiotics in IR procedures has never been proven, and there is a large variation in practice patterns. While the risk of antibiotics to an individual patient is small with potential great benefit, their routine use may breed resistance [47].
The decision to use an antibiotic and the appropriate choice must be based on the most likely potential pathogens. Broad-spectrum antibiotics are inappropriate as they promote resistance that may increase morbidity and mortality [54, 55]. If antibiotics are given, they should be administered less than 2 h prior to the procedure [56, 57]. A single dose may be as effective as multiple doses [47].
While recommendations cannot be made about antibiotics for every procedure, there are some procedures with increased risk of infection. Bile is infected in 2/3 of cases with a benign obstruction and 1/3 of patients with malignant obstruction, with enterococcus the most common pathogen [47]. Biliary stones are often associated with Klebsiella, enterococcus, Pseudomonas and gram-positive cocci. Pyonephrosis may be associated with septic shock in 7% of cases and should be treated as an active urinary tract infection sepsis [47]. For percutaneous cecostomy, different prophylaxis regimens include a combination of ampicillin or cefazolin, gentamicin, and metronidazole [58] (personal correspondence C James), or Ancef (personal correspondence B Connolly). Other percutaneous procedures where the risk of infection is low may be done without antibiotics or with cefazolin for skin pathogen prophylaxis [47].
Infection transmission
While any infection may be transmitted during accidental exposure during an IR procedure, the most worrisome pathogens are HIV, hepatitis B virus (HBV) and hepatitis C virus (HCV) [59]. While the incidence of HIV infection is <1% in all patients, the incidence may be up to 14.2% in some adult hospital settings [59, 60]. HBV is found in approximately 5% of the adult population and HCV in 18% [59, 61]. However, HCV may be present in 83% of IV drug abusers, 21% of transfusion recipients and 19% of patients on hemodialysis [59]. Transmission of HIV, HBV and HCV has never been described through intact skin [59]. The risk of transmission of HIV has been estimated 0.03–7.5/100,000 for a single procedure with a cumulative risk of 0.009–16% over 30 years assuming a 10% risk of each patient having HIV and performing 1,000 procedures/year [62].
The Occupational Safety and Health Association (OSHA) in 1992 enacted a Blood Borne Pathogen Standard into law. It has several components and failure to comply is considered a federal offense [59]. Pathogens include blood and other potentially infected materials (OPIM), including semen, vaginal secretions, saliva and cerebrospinal, synovial, pleural and pericardial fluid. Any other fluid mixed with blood is considered a potential pathogen. Occupational exposure includes parenteral, skin or mucous membrane contact with blood or other potentially infected materials. The concept of universal precautions was developed. In this concept, there can be no eating, drinking, handling of contact lenses or use of cosmetics in work areas. Specimens must be handled and labeled properly, and personal protective equipment must be available. In addition, there must be an exposure control plan available, HBV vaccine must be provided free of charge and a postexposure prophylaxis plan needs to be in place [59].
Hand-washing must be performed before and after each procedure and immediately after removing gloves [59]. Appropriate protective equipment includes gloves, face shield or mask and goggles with side shields, and coverage of non-intact skin with a water-impermeable barrier [59]. Mucous membrane exposure occurs in 31% of IR physicians each year, and in 44% during their careers [61, 63]. If there is a reasonable risk of exposure, a hat, gown, dedicated procedural shoes and shoe covers should be worn [59, 60].
Gloves may develop pinholes in them after a while, and routine glove changes after 90 min may be advisable [59]. If there is broken skin on the hands, the practitioner may use a double glove technique [59].
Needle stick or sharps injuries are the most common cause of pathogen transmission [59]. These injuries are reported in 0.6% of procedures, 38% of IR physicians have a puncture injury each year, and 52% have had a sharps injury during their careers [60, 61, 63]. Seroconversion from a single needle stick occurs in 0.3–0.4% with HIV, up to 30% with HBV and in approximately 3% with HCV [60]. Sharps should not be handed from one person to another or transferred from one hand to another. A needle should never be recapped with a two-hand technique, a sharps holder should be on the table, sharps should be disposed of promptly and a needle holder should always be used when suturing [59, 60].
Summary
Infectious disease is intimately intertwined with pediatric IR. The pediatric IR physician is often asked to diagnose or treat an infection. Unfortunately, we may also be the instigator for patient infection. With proper planning and techniques, we can reduce these risks both to the patient and the health care worker.
References
Price MR, Haase GM, Sartorelli KH et al (1996) Recurrent appendicitis after initial conservative management of appendiceal abscess. J Pediatr Surg 31:291–294
Curran TJ, Muenchow SK (1993) The treatment of complicated appendicitis in children using peritoneal drainage: results from a public hospital. J Pediatr Surg 28:204–208
Pereira JK, Chait PG, Miller SF (1996) Deep pelvic abscesses in children: transrectal drainage under radiologic guidance. Radiology 198:393–396
Alexander AA, Eschelman DJ, Nazarian LN et al (1994) Transrectal sonographically guided drainage of deep pelvic abscesses. AJR 162:1227–1230
Gervais DA, Hahn PF, O’Neill MJ et al (2000) CT-guided transgluteal drainage of deep pelvic abscesses in children: selective uses as an alternative to transrectal drainage. AJR 175:1393–1396
Cahill AM, Kaye RD, Towbin RB et al (2003) Pelvic abscess drainage in children: the trans-gluteal approach. Pediatr Radiol 33(Suppl):S81
Hogan MJ, Hoffer FA (2010) Biopsy and drainage techniques in children. Tech Vasc Interv Radiol 13:206–213
Hogan MJ (2003) Appendiceal abscess drainage. Tech Vasc Interv Radiol 6:205–214
Coley BD, Shiels WE 2nd, Elton S et al (2004) Sonographically guided aspiration of cerebrospinal fluid pseudocysts in children and adolescents. AJR 183:1507–1510
Hogan MJ, Coley BD (2008) Interventional radiology treatment of empyema and lung abscesses. Paediatr Respir Rev 9:77–84
Hawkins JA, Scaife ES, Hillman ND et al (2004) Current treatment of pediatric empyema. Semin Thorac Cardiovasc Surg 16:196–200
Patradoon-Ho P, Fitzgerald DA (2007) Lung abscess in children. Paediatr Respir Rev 8:77–84
Weissberg D (1984) Percutaneous drainage of lung abscess. J Thorac Cardiovasc Surg 87:308–312
Lorenzo RL, Bradford BF, Black J et al (1985) Lung abscesses in children: diagnostic and therapeutic needle aspiration. Radiology 157:79–80
Ball BS, Bisset GS, Towbin RB (1989) Percutaneous drainage of chest abscesses in children. Radiology 171:431–434
Hoffer FA, Bloom DA, Colin AA et al (1999) Lung abscess versus necrotizing pneumonia: implications for interventional therapy. Pediatr Radiol 29:87–91
Cahill AM, Baskin KM, Kaye RD et al (2004) CT-guided percutaneous lung biopsy in children. J Vasc Interv Radiol 15:955–960
Connolly BL, Chait PG, Duncan DS et al (1999) CT-guided percutaneous needle biopsy of small lung nodules in children. Pediatr Radiol 29:342–346
Hoffer FA, Gow K, Flynn PM et al (2001) Accuracy of percutaneous lung biopsy for invasive pulmonary aspergillosis. Pediatr Radiol 31:144–152
Coley BD, Shiels WE 2nd, Hogan MJ (2001) Diagnostic and interventional ultrasonography in neonatal and infant lumbar puncture. Pediatr Radiol 31:399–402
(1995) Quality improvement guidelines for adult percutaneous abscess and fluid drainage. Society of Cardiovascular and Interventional Radiology Standards of Practices Committee. J Vasc Interv Radiol 6:68–70
Wallace MJ, Chin KW, Fletcher TB et al (2010) Quality improvement guidelines for percutaneous drainage/aspiration of abscess and fluid collections. J Vasc Interv Radiol 21:431–435
Rao S, Hogan MJ (2009) Trocar transrectal abscess drainage in children: a modified technique. Pediatr Radiol 39:982–984
Mason KP (2009) Pediatric procedures in interventional radiology. Int Anesthesiol Clin 47:35–43
Koral K, Derinkuyu B, Gargan L et al (2010) Transrectal ultrasound and fluoroscopy-guided drainage of deep pelvic collections in children. J Pediatr Surg 45:513–518
Cremonesini DA, Thomson AH (2007) How should we manage empyema: antibiotics alone, fibrinolytics, or primary video-assisted thoracoscopic surgery (VATS). Semin Respir Crit Care Med 28:322–332
Jaffé A, Balfour-Lynn IM (2005) Management of empyema in children. Pediatr Pulmonol 40:148–156
Stefanutti G, Ghirardo V, Barbato A et al (2010) Evaluation of a pediatric protocol of intrapleural urokinase for pleural empyema: a prospective study. Surgery 148:589–594
Bianchini MA, Ceccarelli PL, Repetto P et al (2010) Once-daily intrapleural urokinase treatment of complicated parapneumonic effusion in pediatric patients. Turk J Pediatr 52:274–277
Schneider CR, Gauderer MW, Blackhurst D et al (2010) Video-assisted thoracoscopic surgery as a primary intervention in pediatric parapneumonic effusion and empyema. Am Surg 76:957–961
Gates RL, Hogan M, Weinstein S et al (2004) Drainage, fibrinolytics, or surgery: a comparison of treatment options in pediatric empyema. J Pediatr Surg 39:1638–1642
Mahant S, Cohen E, Weinstein M et al (2010) Video-assisted thorascopic surgery vs chest drain with fibrinolytics for the treatment of pleural empyema in children: a systematic review of randomized controlled trials. Arch Pediatr Adolesc Med 164:201–203
St Peter SD, Tsao K, Harrison C et al (2009) Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: a prospective, randomized trial. J Pediatr Surg 44:106–111
Sonnappa S, Cohen G, Owens CM et al (2006) Comparison of urokinase and video-assisted thoracoscopic surgery for treatment of childhood empyema. Am J Respir Crit Care Med 174:221–227
Kurt BA, Winterhalter KM, Connors RH et al (2006) Therapy of parapneumonic effusions in children: video-assisted thoracoscopic surgery versus conventional thoracostomy drainage. Pediatrics 118:e547–e553
Meier AH, Hess CB, Cilley RE (2010) Complications and treatment failures of video-assisted thoracoscopic debridement for pediatric empyema. Pediatr Surg Int 26:367–371
Koral K, Saker MC, Morello FP et al (2003) Conventional versus modified technique for percutaneous nephrostomy in newborns and young infants. J Vasc Interv Radiol 14:113–116
Riccabona M, Sorantin E, Hausegger K (2002) Imaging guided interventional procedures in paediatric uroradiology–a case based overview. Eur J Radiol 43:167–179
Babu R, Hutton KA (2004) Renal fungal balls and pelvi-ureteric junction obstruction in a very low birth weight infant: treatment with streptokinase. Pediatr Surg Int 20:804–805
Miller DL, O’Grady NP (2003) Guidelines for the prevention of intravascular catheter-related infections: recommendations relevant to interventional radiology. J Vasc Interv Radiol 14(Pt 1):133–136
O’Grady NP, Alexander M, Dellinger EP et al (2002) Guidelines for the prevention of intravascular catheter-related infections. The Hospital Infection Control Practices Advisory Committee, Center for Disease Control and Prevention, U.S. Pediatrics 110:e51
Gebauer B, El-Sheik M, Vogt M et al (2009) Combined ultrasound and fluoroscopy guided port catheter implantation–high success and low complication rate. Eur J Radiol 69:517–522
Costello JM, Morrow DF, Graham DA et al (2008) Systematic intervention to reduce central line-associated bloodstream infection rates in a pediatric cardiac intensive care unit. Pediatrics 121:915–923
Miller MR, Griswold M, Harris JM 2nd et al (2010) Decreasing PICU catheter-associated bloodstream infections: NACHRI’s quality transformation efforts. Pediatrics 125:206–213
McKee C, Berkowitz I, Cosgrove SE et al (2008) Reduction of catheter-associated bloodstream infections in pediatric patients: experimentation and reality. Pediatr Crit Care Med 9:40–46
Koç O, Peynircioğlu B, Cil BE (2008) Role of culturing from the tip and the tunneled segment of the catheters in tunneled catheter infection. Diagn Interv Radiol 14:228–232
Ryan JM, Ryan BM, Smith TP (2004) Antibiotic prophylaxis in interventional radiology. J Vasc Interv Radiol 15:547–556
Sheridan RL, Weber JM (2006) Mechanical and infectious complications of central venous cannulation in children: lessons learned from a 10-year experience placing more than 1000 catheters. J Burn Care Res 27:713–718
Rayner HC, Besarab A, Brown WW et al (2004) Vascular access results from the Dialysis Outcomes and Practice Patterns Study (DOPPS): performance against Kidney Disease Outcomes Quality Initiative (K/DOQI) Clinical Practice Guidelines. Am J Kidney Dis 44(Suppl 2):22–26
Yamamoto AJ, Solomon JA, Soulen MC et al (2002) Sutureless securement device reduces complications of peripherally inserted central venous catheters. J Vasc Interv Radiol 13:77–81
Cober MP, Kovacevich DS, Teitelbaum DH (2010) Ethanol-lock therapy for the prevention of central venous access device infections in pediatric patients with intestinal failure. JPEN J Parenter Enteral Nutr 35:67–73
Jones BA, Hull MA, Richardson DS et al (2010) Efficacy of ethanol locks in reducing central venous catheter infections in pediatric patients with intestinal failure. J Pediatr Surg 45:1287–1293
Bhutta A, Gilliam C, Honeycutt M et al (2007) Reduction of bloodstream infections associated with catheters in paediatric intensive care unit: stepwise approach. BMJ 334:362–365
Goldman DA, Weinstein RA, Wenzel RP et al (1996) Strategies to prevent and control the emergence and spread of antimicrobial-resistant organisms in hospitals: a challenge to hospital leadership. JAMA 275:234–240
Jones RN, Phaller MA (1998) Bacterial resistance; a worldwide problem. Diagn Microbiol Infect Dis 31:379–388
Burke JF (1961) The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery 50:161–168
Stone HH, Haney BB, Kolb LD et al (1979) Prophylactic and preventive antibiotic therapy: timing, duration, and economics. Ann Surg 189:691–699
Chait PG, Shlomovitz E, Connolly BL et al (2003) Percutaneous cecostomy: updates in technique and patient care. Radiology 227:246–250
Hansen ME, Bakal CW, Dixon GC et al (2003) Society of Interventional Radiology Technology Assessment Committee. Guidelines regarding HIV and other bloodborne pathogens in vascular/interventional radiology. J Vasc Interv Radiol 14(Pt 2):S375–S384
Hansen ME (1998) Bloodborne pathogens and procedure safety in interventional radiology. Semin Ultrasound CT MR 19:209–214
Marx MV (2003) Hepatitis C virus risk in the interventional radiology environment. J Vasc Interv Radiol 14(Pt 1):129–131
Hansen ME, McIntire DD (1996) HIV transmission during invasive radiologic procedures: estimate based on computer modeling. AJR 166(2):263–267
Baffoy-Fayard N, Maugat S, Sapoval M et al (2003) Study Group on Hygiene Practices in Interventional Radiology. Potential exposure to hepatitis C virus through accidental blood contact in interventional radiology. J Vasc Interv Radiol 14(2 Pt 1):173–179
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclaimer The supplement this article is part of is not sponsored by the industry. Dr. Hogan has no financial interests, investigational or off-label uses to disclose.
Rights and permissions
About this article
Cite this article
Hogan, M.J. Infection in pediatric interventional radiology. Pediatr Radiol 41 (Suppl 1), 99–106 (2011). https://doi.org/10.1007/s00247-011-2000-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-011-2000-z