Abstract
Background
Cocaine exposure during pregnancy has been reported to have detrimental effects on the fetus.
Objective
To describe the findings on cranial ultrasonography (CUS) as part of a neonatal screening programme for exposed neonates.
Materials and methods
The study was a semiprospective analysis of a 12-year cohort of neonates born to mothers who had used cocaine during their pregnancy and who had follow-up according to a strict clinical protocol.
Results
In total, 154 neonates (78 boys, 76 girls) were included, of whom 29 (19%) were born preterm, and 125 (81%) were born full-term. Abnormalities on CUS were seen in 37 neonates (24%; 95% CI 18–31%). The abnormalities were classified as minor in 20 (13%; 95% CI 9–19%) and mildly abnormal in 17 (11%; 95% CI 7–17%). None of the infants showed severe abnormalities. The abnormalities were not associated with the duration or maximum amount of cocaine use during pregnancy.
Conclusion
None of the infants had severe abnormalities. Detected abnormalities were not correlated with the duration or maximum amount of cocaine use. Given these findings, we feel that routine cranial ultrasonography in this population is not warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the late 1980s intrauterine cocaine exposure has been reported to have detrimental effects on the fetus and to be a risk for pregnancy and delivery [1, 2]. Currently, cocaine is one of the most popular illicit drugs (besides marijuana) in the Netherlands, being used in all socioeconomic groups. Cocaine has been specifically implicated as a drug with major side effects on pregnancy. In their seminal paper, Dixon and Bejar [3] reported a sevenfold increase in abnormalities on cranial ultrasonography (CUS) in general and a twofold increase in the incidence of subependymal haemorrhage (SEH) in neonates exposed to cocaine and/or methamphetamine. In a relatively small study amongst 21 cocaine users in Amsterdam a 4.3% neonatal mortality rate was found [4].
Cocaine has two major pharmacological effects. First, it inhibits the reuptake of dopamine, norepinephrine and serotonin. Second, it causes vasoconstriction. As cocaine crosses the placenta, obstetric implications of cocaine use have been reported. These implications are an increased risk of placental abruption [5–7], preterm prelabour rupture of membranes [8], and spontaneous abortion. In the neonate, detrimental effects on brain development have been reported [3, 9, 10]. These effects include, amongst others, infarction [11], subependymal cysts [12, 13], haemorrhage, ventricular dilatation, and midline CNS defects [11]. Besides intracranial pathology, congenital malformations, intrauterine growth restriction [14], and neonatal abstinence syndrome [15] have also been reported.
As a result of reports on the effect of cocaine on brain development, the Departments of Obstetrics and Gynaecology, Neonatology and Radiology of the Academic Medical Center Amsterdam instituted screening CUS in neonates with prenatal cocaine exposure. However, more recent reviews of the literature indicated that antenatal cocaine exposure has measurable, but not dramatic effects on infant brain development and CUS studies [16, 17].
The aim of the present semiprospective study was to assess the frequency and severity of abnormalities on CUS, and to assess whether there is an association between the maximum amount or duration of cocaine use during pregnancy.
Materials and methods
This was a semiprospective study. Consecutive neonates were prospectively included if maternal cocaine use during pregnancy was reported, and CUS was retrospectively analysed. The study was part of a larger semiprospective cohort study of maternal illicit drug users in the greater Amsterdam region. The Department of Obstetrics of the Academic Medical Centre, Amsterdam, led the study. The inclusion period for this retrospective study ran from 1 January 1995 to 31 December 2006.
Participants
All participants enrolled in this semiprospective study came from a cohort of consecutive neonates born between 1 January 1995 and 31 December 2006 to mothers who used at least cocaine during pregnancy and who were referred to our outpatient obstetric department. Women using cannabis, opiates and methadone, and other illicit drugs besides cocaine were not excluded. Drug use was assessed through extensive drug history, urine screening of the mother, and urine screening of the child. The amount of cocaine use was scored as intermittent (i.e. not daily during pregnancy; usually <1 g per occasion), <1 g daily, 1–4 g daily, and >4 g daily. Duration of cocaine use was scored as only in the first trimester, in the first and second trimesters, or throughout pregnancy.
If, in any trimester, the mother had a positive history or positive urine test for cocaine or if the neonate had a positive urine test for cocaine, both mother and child were included in the study. In addition, during pregnancy women using cocaine were structurally interviewed by the same midwife (M.v.H.) with respect to quality and quantity of illicit drug use and life-style in each trimester. Clinical data were further obtained through review of medical charts of both mothers and neonates.
In light of the question posed in this study, only those neonates who underwent CUS and whose CUS was considered of sufficient quality for review were analysed.
Cranial ultrasonography
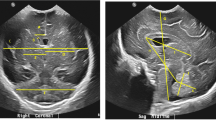
CUS was performed following the standard routine in place during the study period. Examinations were performed by radiology residents, both with and without supervision of a paediatric radiologist, and paediatric radiologists. CUS was performed using a 7.5-MHz curvilinear transducer and contemporary state-of-the-art equipment. As the studies were performed in a clinical setting there were no specified imaging planes.
CUS studies were retrospectively assessed by one paediatric radiologist (R.v.R.). Although the reviewer was aware of the fact that all studies submitted for review were of neonates with prenatal exposure to cocaine, he was not aware of any clinical findings, maternal nor neonatal, or clinical outcome of the neonates.
The majority of the CUS studies were reviewed on a PACS system (AGFA Impax 4.1 SP4 SU4; Agfa Gevaert, Mortsel, Belgium). A minority of the studies were printed on film and these were of technically adequate quality to review.
Intraventricular haemorrhages were graded according to the procedure of Papile et al. [18]. Increased periventricular echogenicity was scored as either present (periventricular leukomalacia, PVL, grade I) or absent; cystic degeneration was scored as either local (PVL grade II) or disseminated (PVL grade III). Ventricular dilatation was assessed using the Levene index in neonates up to 40 weeks’ gestational age (GA) and using the ventricular index in older neonates [19, 20]. The scans were also re-evaluated for the presence of lenticulostriate vasculopathy, calcifications, germinal layer cysts, choroid plexus cysts, and subependymal pseudocysts.
CUS was scored according to procedure of Rademaker et al. [21] and was considered to be normal when no or minor abnormalities, such as germinal layer/plexus cysts, subependymal pseudocysts, or calcifications as exclusive findings were present; mildly abnormal when a haemorrhage grade I or II according to Papile, PVL I, or germinal layer necrosis, or a combination of these features, was present; and severely abnormal when one or more of the following features were present: haemorrhage grade III or IV according to Papile, cystic PVL II/III, thalamic lesion, focal infarction, or convexity haemorrhage.
Statistical analysis
Statistical analysis was performed using SPSS for Windows 12.0. (Statistical Package for the Social Sciences, Chicago, IL). Results for normally distributed continuous variables (birth weight, GA, etc.) are expressed as means±SD. For the neonates, growth data at birth were analysed with the “Kloosterman growth chart” in order to calculate the standard deviation score (SDS) [22].
Bivariate correlations were analysed using a nonparametric test. The following correlations were tested: CUS abnormalities (classified according to Rademaker et al. [21]) versus duration and amount of cocaine use, maternal age, maternal education level, number of prenatal visits, co-use of other drugs, alcohol or smoking, complications during pregnancy (intrauterine growth retardation, preeclampsia, HELLP syndrome, or premature labour), complications during delivery (fetal distress, meconium-stained amniotic fluid, premature rupture of membranes, prolonged rupture of membranes, or perinatal infection), GA, birth weight (in grams and expressed as SDS), sex, and 1- and 5-min Apgar scores. P values <0.05 were assumed to reflect statistical significance.
Ethical approval
According to Academic Medical Center guidelines for retrospective studies, internal review board approval is not required for the retrospective analysis of clinically obtained data.
Results
General
Between 1 January 1995 and 31 December 2006, 188 neonates (97 boys, 52%; 91 girls, 48%) were born to 178 women who had used cocaine during pregnancy. Of these women, six had had two pregnancies, one had had three pregnancies, and two delivered twins. The mean age of the mothers at the time of delivery was 29.3 years (SD 6.2 years, range 18.2–42.4 years).
In 15 (8%) of the 188 neonates there was a protocol violation as CUS was not performed. Of the remaining 173, 19 (11%) CUS studies were of insufficient quality for review and were excluded from further analysis. Of the examinations of insufficient quality for review, 12 were performed by radiology residents (three, 16%, supervised and nine, 47%, unsupervised), and seven (37%) were performed by a radiologist. There were no significant differences between neonates with a CUS examination considered to be of sufficient quality for review and those with a CUS examination considered to be of insufficient quality for review with respect to maternal age (P=0.094), GA (P=0.308), birth weight (P=0.226), birth weight SDS (P=0.735), sex distribution (P=0.264), duration (P=0.298), or amount of cocaine use (P=0.929). Review of the radiology reports of the 19 ‘insufficient quality’ examinations revealed no intracranial pathology in 15 neonates (79%; 95% CI 56–92%), in three of which repeat CUS was performed at respectively 1, 3 and 5 months after the initial CUS, and these CUS were also reported to be normal. In four neonates (21%; 95% CI 8–44%) abnormalities were seen; in one a choroid plexus cyst and in the other three grade 1 SEH.
In total, 154 CUS studies of good quality (82%, 78 boys and 76 girls, born to 147 mothers) were available for review, and were included in all further analyses.
Maternal characteristics
In 52 (34%) of the 154 pregnancies, the mother used cocaine in the first trimester only, in 11 (7%) in the first and second trimester only, and in 87 (56%) also during the third trimester. Two women had a positive urine test in the third trimester only; all other urine tests and histories were negative for cocaine use. Two children had a positive urine test, although the history and/or urine test of the mothers were negative. In 25 (16%) of the pregnancies, the amount of cocaine used by the mother was not known; in 37 (24%) the mother used it intermittently, in 59 (38%) the mother used <1 g daily, in 24 (16%) the mother used 1–4 g daily, and in 9 (6%) the mother used >4 g daily.
Co-use of alcohol and nicotine was common in the study population. Alcohol use during pregnancy was recorded in 94 women; 53 (56%) used alcohol throughout pregnancy. Smoking during pregnancy was recorded in 137 women; 130 (95%) smoked cigarettes throughout pregnancy. Co-use of other drugs is shown in Table 1.
Child characteristics
Nine neonates (6%; 95% CI 3–11%) were born before 33 weeks’ GA, 20 (13%; 95% CI 8–19%) between 33 and 37 weeks’ GA, and 125 (81%; 95% CI 74–87%) from 37 weeks’ GA onwards. The mean birth weight was 2,890±643 g (range 780–4,350 g); the mean birth weight SDS was −0.62±0.89 (range −2.72 to +2.18). Of the 154 neonates, 36 (23%; 95% CI 17–31%) were small for GA (birth weight <−1.3 SDS), and 4 newborns (3%; 95% CI 1–7%) were large for GA (birth weight >+1.3 SDS). The median Apgar score after 1 min was 9 (range 1–10) and after 5 min was 10 (range 4–10). There were no neonatal deaths in our study cohort (two fetal deaths at 35 and 36 weeks are not included).
Cranial ultrasonography
Cranial ultrasonography was performed by radiology residents in 79 cases (51%), in 37 cases with supervision, and in 42 cases without supervision; the remaining 75 CUS (49%) were performed by radiologists. CUS was performed on average on day 4 (range 0–76). Thirty-seven out of 154 sufficient-quality CUS (24%; 95% CI 18–31%) showed abnormalities on the CUS. Seven of these neonates were born at a GA of ≤32 weeks (19%; 95% CI 9–35%), 8 between 33 and 37 weeks (22%; 95% CI 11–37%) and 22 full term (60%; 95% CI 43–74%).
Table 2 shows the abnormalities on CUS for three GA groups. In total there were 16 grade I SEH in 13 neonates. Of these 13 neonates, 8 (22%; 95% CI 11–37%) showed unilateral grade 1 SEH and 2 (5%; 95% CI 1–19%) showed bilateral grade 1 SEH. One neonate (3%; 95% CI 0–15%) showed a right-sided grade 2 SEH and a left-sided grade 1 SEH. Two neonates (5%; 95% CI 1–19%) showed cystic (old) SEH, one of them bilateral. In 13 out of 37 neonates (35%; 95% CI 22–51%) a choroid plexus cyst was recorded on CUS. Three neonates had a subependymal cyst (8%; 95% CI 2–22%). Five neonates showed increased periventricular echogenicity (14%; 95% CI 5–28%), two of them bilateral. Three neonates had unilateral lenticulostriate vasculopathy (8%; 95% CI 2–22%), and in five it was bilateral (14%; 95% CI 5–28%). One neonate (3%; 95% CI 0–15%) showed mild bilateral ventricular dilatation without other abnormalities.
Applying the criteria of Rademaker et al. [21], the abnormalities could be classified as minor in 20 (13%; 95% CI 8–19%) neonates and mildly abnormal in 17 (11%; 95% CI 7–17%). None of the infants showed severe abnormalities.
Bivariate correlations showed that neither the duration nor the maximum amount of cocaine use during pregnancy was correlated with CUS abnormalities (Table 3). In addition, co-use of other drugs, alcohol use, or cigarette smoking did not correlate with CUS abnormalities. CUS abnormalities were found more frequently with fewer prenatal visits after 20 weeks’ GA, in complicated pregnancies, shorter duration of GA, and lower absolute birth weight (Table 3).
Discussion
Neonatal congenital anomalies seen on CUS have been related to prenatal cocaine exposure [13, 23, 24]. In our study population 24% of the neonates with antenatal cocaine exposure showed abnormalities on CUS. Although this seems high, it must be noted that all abnormalities could be classified as minor (13%) or mild (11%); none of the infants had severe CUS abnormalities. In addition, CUS abnormalities were not correlated with the duration or maximum amount of cocaine use.
The literature shows conflicting data with regard to prenatal cocaine exposure in relation to CUS findings. Smith et al. [13] found an increased incidence of subependymal cysts in neonates with prenatal cocaine exposure compared to non-exposed neonates (44% vs. 8%, P<0.01) [13]. Dogra et al. [9] found abnormalities on CUS in 14 of 40 (35%) neonates, with prenatal cocaine exposure, whereas their age-matched control group showed no abnormalities on CUS. The main findings were focal echolucencies (i.e. cystic lesions) and increased echogenicity of the caudate nucleus.
In a study by King et al. [25], however, no significant difference was found between exposed neonates (incidence 24%) and non-exposed neonates (incidence 34%) with respect to abnormalities found on CUS. These findings are supported by Bauer et al. [15] who compared CUS in 132 exposed neonates to 711 non-exposed neonates. In the exposed group 29.5% of the neonates and in the non-exposed groups 30.8% of the neonates showed abnormalities on CUS. As part of the only prospective longitudinal study published to date, 154 cocaine users were matched to 154 control subjects; no significant difference between these groups in the incidence of abnormal CUS (13% in exposed vs. 8% in non-exposed neonates) was found [26]. Furthermore, the authors specifically stated that the identified lesions were less severe than previously reported.
There are few data on the influence of the level of cocaine exposure on CUS abnormalities. Only one study assessed a dose-effect relationship between prenatal cocaine exposure and CUS findings [27]. They showed that infants with heavier cocaine exposure were more likely to show SEH than lighter or unexposed infants (adjusted odds ratio 3.88; 95% CI 1.45–10.35). This is in contrast to our results. We performed a subgroup analysis, but this also did not reveal a correlation between SEH and the maximum amount or duration of cocaine use in pregnancy.
An important factor that has received relatively little attention is the relationship between neonatal CUS findings and neurobehavioural outcome in (near-)term infants with antenatal cocaine exposure. Behnke et al. [28] studied 266 infants, both with and without prenatal exposure to cocaine, who underwent CUS within the first 96 h after birth. There were 239 normal examinations and 27 abnormal examinations. Based on the Amiel-Tison neonatal neurological assessment, performed within 96 h, there was no significant difference between the two groups. Neurodevelopmental outcome has been studied in preterm infants who underwent screening CUS. Rademaker et al. [21] studied a 2-year cohort of 221 preterm infants and related neonatal CUS findings to school performance. Children with normal or mildly abnormal CUS had similar mean IQ values and the proportion of children with an IQ ≤85 was not different between these CUS groups. Pisani et al. [29] followed 78 preterm infants without CUS abnormalities and 50 preterm infants with transient periventricular echodensities up to the age of 12 months [29]. At 12 months, 88.5% of the first group and 94% of the second group showed normal neurodevelopment. They concluded that the two groups showed identical outcomes with regard to neurodevelopment. In a study by Stewart et al. [30] of very preterm neonates, 5 of 62 infants (8%) with normal CUS and 2 of 25 infants (8%) with uncomplicated periventricular haemorrhage showed neurological or developmental sequelae at 18 months of age.
As in all clinical studies with drug abusers, confounding factors are abundant. In general, socioeconomic status is lower than in the general population. In The Netherlands we generally have a more liberal approach to illicit drug use, which allows early medical support and intervention during pregnancy. This might reduce this effect somewhat. A second confounding factor is the co-use of nicotine, alcohol and other illicit drugs besides cocaine. Teratogenic effects on the developing brain have been described in maternal nicotine and alcohol use. In case of maternal nicotine use, a study in rats has shown that on a cellular level nicotinic cholinergic receptor expression is altered [31]. However, in these cases no gross developmental abnormalities have been described. In prenatal alcohol exposure a reduction in the frontal cortex has been described. This study using CUS as the imaging modality does not allow evaluation of this anomaly [32].
As this was a semiprospective study it had inherent drawbacks. First, US is an operator-dependent technique that makes review a difficult task. Over time, different US systems have been used in our hospital, all reflecting the state-of-the-art at the time of use. As the studies were performed in a clinical setting there were no specified imaging planes for CUS. This led to 19 examinations, containing only a limited number of images, being regarded as inadequate for inclusion in the study. The authors assumed that if at the time of the examination an ultrasonographer had seen an abnormality he/she would likely have stored more images than in an otherwise normal study. Based on this assumption a separate analysis of the US reports in these 19 neonates was performed. This showed that exclusion of these cases introduced no bias into our study.
A second drawback is the lack of a control group. We did not attempt to include a control group because we felt it was impossible to compose a group that was completely comparable, especially in life-style and socioeconomic background, to drug users. We therefore had to compare our findings to the findings reported in the literature. Third, it is well known that reliable assessment of drug use is extremely difficult. This obviously has implications for the evaluation of a dose-effect relationship.
Our findings on the occurrence of CUS abnormalities after prenatal cocaine exposure are in keeping with previously published papers [15, 25, 26]. However, it is known that at a later age children with prenatal cocaine exposure may show neurocognitive development disorders. To further study these effects of prenatal cocaine exposure at a later age functional MRI in exposed adolescents, matched to a non-exposed population, would be worth considering.
Although the frequency of CUS abnormalities seems to be high in our study population (around a quarter of the neonates), it corresponds with non-exposed infants in recent reports in the literature. All abnormalities were classified as minor or mild and would probably have no long-term consequences for neurodevelopment. In addition, abnormalities detected with CUS were not correlated with the duration or maximum amount of cocaine use. In spite of the semiprospective nature of our study, we feel on the basis of our findings, in combination with data presented in the literature, that CUS screening in this specific population is not warranted.
References
Boer K, Smit BJ, van Huis AM et al (1994) Substance use in pregnancy: do we care? Acta Paediatr Suppl 404:65–71
Holzman C, Paneth N (1994) Maternal cocaine use during pregnancy and perinatal outcomes. Epidemiol Rev 16:315–334
Dixon SD, Bejar R (1989) Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: incidence and clinical correlations. J Pediatr 115:770–778
Smit BJ, Boer K, van Huis AM et al (1994) Cocaine use in pregnancy in Amsterdam. Acta Paediatr Suppl 404:32–35
Townsend RR, Laing FC, Jeffrey RB Jr (1988) Placental abruption associated with cocaine abuse. AJR 150:1339–1340
Landy HJ, Hinson J (1987) Placental abruption associated with cocaine use: case report. Reprod Toxicol 1:203–205
Hulse GK, Milne E, English DR et al (1997) Assessing the relationship between maternal cocaine use and abruptio placentae. Addiction 92:1547–1551
Addis A, Moretti ME, Ahmed SF et al (2001) Fetal effects of cocaine: an updated meta-analysis. Reprod Toxicol 15:341–369
Dogra VS, Shyken JM, Menon PA et al (1994) Neurosonographic abnormalities associated with maternal history of cocaine use in neonates of appropriate size for their gestational age. AJNR 15:697–702
Gomez-Anson B, Ramsey RG (1994) Pachygyria in a neonate with prenatal cocaine exposure: MR features. J Comput Assist Tomogr 18:637–639
Heier LA, Carpanzano CR, Mast J et al (1991) Maternal cocaine abuse: the spectrum of radiologic abnormalities in the neonatal CNS. AJNR 12:951–956
Cohen HL, Sloves JH, Laungani S et al (1994) Neurosonographic findings in full-term infants born to maternal cocaine abusers: visualization of subependymal and periventricular cysts. J Clin Ultrasound 22:327–333
Smith LM, Qureshi N, Renslo R et al (2001) Prenatal cocaine exposure and cranial sonographic findings in preterm infants. J Clin Ultrasound 29:72–77
Chazotte C, Youchah J, Freda MC (1995) Cocaine using during pregnancy and low birth weight: the impact of prenatal care and drug treatment. Semin Perinatol 19:293–300
Bauer CR, Langer JC, Shankaran S et al (2005) Acute neonatal effects of cocaine exposure during pregnancy. Arch Pediatr Adolesc Med 159:824–834
Frank DA, Augustyn M, Zuckerman B (1998) Neonatal neurobehavioral and neuroanatomic correlates of prenatal cocaine exposure. Problems of dose and confounding. Ann N Y Acad Sci 21:40–50
Frank DA, Augustyn M, Knight WG et al (2001) Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review. JAMA 285:1613–1625
Papile LA, Burnstein J, Burnstein R et al (1978) Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92:529–534
Levene MI (1981) Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child 56:900–904
Shah PS, Sarvaiya JB, Rawal JR et al (1992) Normal ventricular size and ventriculo-hemispheric ratio in infants up to 6 months of age by cranial ultrasonography. Indian Pediatr 29:439–442
Rademaker KJ, Uiterwaal CS, Beek FJ et al (2005) Neonatal cranial ultrasound versus MRI and neurodevelopmental outcome at school age in children born preterm. Arch Dis Child Fetal Neonatal Ed 90:F489–F493
Kloosterman GJ (1970) On intrauterine growth. Int J Gynaecol Obstet 8:895–912
Chasnoff IJ, Bussey ME, Savich R et al (1986) Perinatal cerebral infarction and maternal cocaine use. J Pediatr 108:456–459
Haataja L, Mercuri E, Cowan F et al (2000) Cranial ultrasound abnormalities in full term infants in a postnatal ward: outcome at 12 and 18 months. Arch Dis Child Fetal Neonatal Ed 82:F128–F133
King TA, Perlman JM, Laptook AR et al (1995) Neurologic manifestations of in utero cocaine exposure in near-term and term infants. Pediatrics 96:259–264
Behnke M, Davis EF, Conlon M et al (1998) Incidence and description of structural brain abnormalities in newborns exposed to cocaine. J Pediatr 132:291–294
Frank DA, McCarten KM, Robson CD et al (1999) Level of in utero cocaine exposure and neonatal ultrasound findings. Pediatrics 104:1101–1105
Behnke M, Eyler FD, Garvan CW et al (1999) Cranial ultrasound abnormalities identified at birth: their relationship to perinatal risk and neurobehavioral outcome. Pediatrics 103:e41
Pisani F, Leali L, Moretti S et al (2006) Transient periventricular echodensities in preterms and neurodevelopmental outcome. J Child Neurol 21:230–235
Stewart AL, Thorburn RJ, Hope PL et al (1983) Ultrasound appearance of the brain in very preterm infants and neurodevelopmental outcome at 18 months of age. Arch Dis Child 58:598–604
Chen H, Parker SL, Matta SG et al (2005) Gestational nicotine exposure reduces nicotinic cholinergic receptor (nAChR) expression in dopaminergic brain regions of adolescent rats. Eur J Neurosci 22:380–388
Wass TS, Persutte WH, Hobbins JC (2001) The impact of prenatal alcohol exposure on frontal cortex development in utero. Am J Obstet Gynecol 185:737–742
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van Huis, M., van Kempen, A.A.M.W., Peelen, M. et al. Brain ultrasonography findings in neonates with exposure to cocaine during pregnancy. Pediatr Radiol 39, 232–238 (2009). https://doi.org/10.1007/s00247-008-1079-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-008-1079-3