Abstract
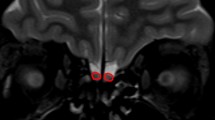
Background: There is limited knowledge of the MRI pattern of the development of fetal olfactory bulbs and sulci. Objective: To describe the MRI appearance of olfactory bulbs and sulci in normal in vivo fetuses according to gestational age. Materials and methods: Olfactory bulbs and sulci were retrospectively assessed on brain MRI examinations of 88 normal fetuses between 24 and 39 weeks gestational age. Two reference centres were involved in the study and both used routine protocols that included axial and coronal T2- and T1-weighted sequences at 1.5 T. The results were compared both with the commonly used neuropathological data in the literature and with personal neuropathological data. Pearson’s chi-squared test or Fisher’s exact test were performed. One case of olfactory agenesis associated with CHARGE syndrome was identified. Results: T2-weighted coronal sequences were the most sensitive for detecting olfactory bulbs and sulci. Olfactory sulci were significantly better detected from 30 weeks onwards (90.9–100%; P<0.001). MRI showed a posteroanterior development of these sulci. Olfactory bulbs were better detected from 30 to 34 weeks (80–90.9%; P<0.002). Comparison with neuropathological data confirmed the posteroanterior development of the sulci and showed an important delay in detection of the olfactory structures (bulbs and sulci). No difference was observed between the two centres involved. Conclusions: To date, fetal MRI can depict olfactory sulci from 30 weeks gestational age onwards and olfactory bulbs from 30 to 34 weeks gestational age. This preliminary reference standard is useful to assess the normality of the olfactory system and to diagnose olfactory agenesis.









Similar content being viewed by others
References
Smith FW, Adam AH, Phillips WD (1983) NMR imaging in pregnancy. Lancet 1:61–62
Merzoug V, Ferey S, Andre C, et al (2002) Magnetic resonance imaging of the fetal brain. J Neuroradiol 29:76–90
Girard N, Raybaud C, Gambarelli D, et al (2001) Fetal brain MR imaging. Magn Reson Imaging Clin N Am 9:19–56
Coakley FV, Glenn OA, Qayyum A, et al (2004) Fetal MRI: a developing technique for the developing patient. AJR Am J Roentgenol 182:243–252
Girard N (2002) Fetal MR imaging. Eur Radiol 12:1869–1871
Kiefer B, Grassner J, Haussman R (1994) Image acquisition in a second with half Fourier acquisition single-shot turbo spin-echo. J Magn Reson Imaging 4:86–87
Suski M, Takashima T, Kadoya M, et al (1989) MR imaging of olfactory bulbs and tract. AJNR Am J Neuroradiol 10:955–957
Youssem DM, Oguz KK, Li C (2001) Imaging of the olfactory system. Semin Ultrasound CT MR 22:456–472
Held P, Seitz J, Fründ R, et al (2000) MRI detection of olfactory bulb and tract. J Neuroradiol 27:112–118
Castillo M (2002) Imaging of the upper cranial nerves I, III–VII, and the cavernous sinuses. Magn Reson Imaging Clin N Am 10:415–431
Truwit CL, Barkovich AJ, Grumbach MM, et al (1993) MR imaging of Kallmann syndrome, a genetic disorder of neuronal migration affecting the olfactory and genital systems. AJNR Am J Neuroradiol 14:827–838
Knorr JR, Ragland RL, Brown RS, et al (1993) Kallmann syndrome: MR findings. AJNR Am J Neuroradiol 14:845–851
Yousem DM, Turner WJ, Li C, et al (1993) Kallmann syndrome: MR evaluation of olfactory system. AJNR Am J Neuroradiol 14:839–843
Yousem DM, Geckle RJ, Bilker W, et al (1996) MR evaluation of patients with congenital hyposmia or anosmia. AJR Am J Roentgenol 166:439–443
Fuerxer F, Carlier R, Iffenecker C, et al (1996) Magnetic resonance imaging of the olfactory sulci in Kallmann syndrome. J Neuroradiol 23:223–230
Klingmuller D, Dewes W, Krahe T, et al (1987) Magnetic resonance imaging of the brain in patients with anosmia and hypothalamic hypogonadism (Kallmann’s syndrome). J Clin Endocrinol Metab 65:581–584
Bajaj S, Ammini AC, Marwaha R, et al (1993) Magnetic resonance imaging of the brain in idiopathic hypogonadotropic hypogonadism. Clin Radiol 48:122–124
Abolmaali ND, Hietschold V, Vogl TJ, et al (2002) MR evaluation in patients with isolated anosmia since birth or early childhood. AJNR Am J Neuroradiol 23:157–164
Cheng L, Youssem DM, Doty RL, et al (1994) Neuroimaging in patients with olfactory dysfunction. AJR Am J Roentgenol 162:411–418
Garel C, Chantrel E, Brisse H, et al (2001) Fetal cerebral cortex: normal gestational landmarks identified using prenatal MR imaging. AJNR Am J Neuroradiol 22:184–189
Naidich TP, Grant JL, Altman N, et al (1994) The developing cerebral surface. Preliminary report on the patterns of sulcal and gyral maturation—anatomy, ultrasound, and magnetic resonance imaging. Neuroimaging Clin N Am 4:201–240
Parent A, Carpenter MB (1996) Carpenter’s human neuroanatomy, 9th edn. Williams and Wilkins, Baltimore, pp 748–756
Chi JG, Dooling EC, Gilles FH (1970) Gyral development of the human brain. Ann Neurol 1:86–93
Adamsbaum C, Merzoug V, Andre Ch, et al (2002) Prenatal diagnostic of isolated posterior fossa anomalies: attempt at a simplified approach (in French). J Radiol 83:321–328
Adamsbaum C, Moutard ML, Andre C, et al (2005) MRI of the fetal posterior fossa. Pediatr Radiol 35:124–140
Garel C (2004) MRI of the fetal brain. Springer, Berlin Heidelberg New York, pp 217–236
Hummel T, Damm M, Vent J, et al (2003) Depth of olfactory sulcus and olfactory function. Brain Res 975:85–89
Bossy J (1980) Development of olfactory and related structures in staged human embryos. Anat Embryol 161:225–236
Humphrey T (1940) The development of the olfactory and accessory formations in human embryos and fetus. J Comp Neurol 73:431–468
Lan LM, Yamashita Y, Tang Y, et al (2000) Normal fetal brain development: MR imaging with a half-Fourier rapid acquisition with relaxation enhancement sequence. Radiology 215:205–210
Vandermarcq P, Sakka M, Lapierre L (1996) Morphologie évolutive du lobe olfactif chez l’homme et quelques mammifères non humains. Chirurgie 121:241–252
Schwanzel-Fukuda M, Bick D, Pfaff DW (1989) Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res 6:311–326
Schwanzel-Fukuda M, Pfaff DW (1989) Origin of luteinizing hormone-releasing hormone neurons. Nature 338:161–164
Cohen MM Jr (2002) Malformations of the craniofacial region: evolutionary, embryonic, genetic, and clinical perspectives. Am J Med Genet 115:245–268
Cohen MM Jr, Shiota K (2002) Teratogenesis of holoprosencephaly. Am J Med Genet 109:1–15
Tellier AL, Amiel J, Delezoide AL, et al (2000) Expression of the PAX2 gene in human embryos and exclusion in the CHARGE syndrome. Am J Med Genet 93:85–88
Lin AE, Siebert JR, Graham JM Jr (1990) Central nervous system malformations in the CHARGE association. Am J Med Genet 37:304–310
Acknowledgements
We acknowledge the contribution of Mme. Lenat and Mme. Trahand for corrections to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was awarded the“Jean François GINESTY” prize by the French Academy of Medicine
Rights and permissions
About this article
Cite this article
Azoulay, R., Fallet-Bianco, C., Garel, C. et al. MRI of the olfactory bulbs and sulci in human fetuses. Pediatr Radiol 36, 97–107 (2006). https://doi.org/10.1007/s00247-005-0030-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-005-0030-0