Abstract
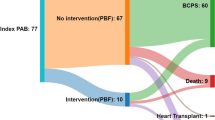
After bidirectional cavopulmonary connection (BDCPC) central pulmonary arteries (PAs) of single ventricle (SV) patients can be affected by stenosis or even closure. Aim of this study is to compare SV patients with and without PA-stent implantation post-BDCPC regarding risk factors for stent implantation and outcome. Single center, retrospective (2006–2021) study of 136 SV consecutive patients with and without PA-stent implantation post-BDCPC. Patient characteristics, risk factors for PA-stent implantation and PA growth were assessed comparing angiographic data pre-BDCPC and pre-TCPC. A total of 40/136 (29%) patients underwent PA-stent implantation at median (IQR) 14 (1.1–39.0) days post-BDCPC. 37/40 (92.5%) underwent LPA-stenting. Multiple regression analysis showed single LV patients to receive less likely PA-stents than single RV patients (OR 0.41; p = 0.05). Reduced LPA/BSA (mm/m2) and larger diameter of neo-ascending aorta pre-BDCPC were associated with an increased likelihood of PA-stent implantation post-BDCPC (OR 0.89, p = 0.03; OR 1.05, p = 0.001). Stent re-dilatation was performed in 36/40 (89%) after 1 (0.8–1.5) year. Pulmonary artery diameters pre-BDCPC were lower in the PA-stent group: McGoon (p < 0.001), Nakata (p < 0.001). Indexed pulmonary artery diameters increased equally in both groups but remained lower pre-TCPC in the PA-stent group: McGoon (p < 0.001), Nakata (p = 0.009), and Lower Lobe Index (p = 0.003). LPA and RPA grew symmetrically in both groups. Single RV, larger neo-ascending aorta, and small LPA pre- BDCPC are independent risk factors for PA-stent implantation post-BDCPC. Pulmonary artery diameters after PA-stent implantation and stent re-dilatation showed significant growth together with the contralateral side, but the PA-system remained symmetrically smaller in the stent group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary artery growth, a sufficient cross-sectional area, and low resistance of the pulmonary vascular system strongly influence long-term outcome of patients with single ventricle (SV) physiologies undergoing Fontan completion [1,2,3,4].
Vessel stenosis, vessel distortion, vessel obstruction, or hypoplasia of the central and peripheral pulmonary arteries (PAs), especially of the left pulmonary artery (LPA), affect almost half of the SV patients after bidirectional cavopulmonary connection (BDCPC) [5,6,7,8,9,10]. This may be attributed to an obstruction, which is due to external compression by the reconstructed aorta (anteriorly) or left main bronchus (posteriorly) [7, 8, 11, 12], or a stenosis, which results from abnormal surgical connections, ductal constriction, or as a consequence of surgical patch reconstruction during BDCPC [4].
Since a certain degree of compression is almost always present [7, 8, 11, 12], surgical management of these lesions may be limited by the proximity to the surrounding structures [7]. On the other hand, a PA-stent fixes the branch PA to a static non-pulsatile and non-compressible state [8, 12]. Percutaneous PA-stent implantation has become the preferred treatment option for these patients, even in the early postoperative period [5, 12,13,14,15,16,17]. Nevertheless, the stent implanted during infancy does not grow and needs to be re-dilated or even removed surgically during follow up [6,7,8].
Improvement of the size and flow to the PAs, even in mild cases of stenosis or mild compression, can improve the hemodynamics and potentially maximize the potential for future symmetrical growth of the PAs by equalizing blood flow to both lungs [7, 18]. Therefore prompt relief of pulmonary stenosis is essential [5, 6]. Identifying risk factors, ideally making a prediction of patients at risk for later PA-stent implantation, as well as timely recognition, and interventional therapy of these lesions, potentially also as a hybrid procedure during BDCPC, may significantly improve outcome.
Little has been published on PA-stent implantation after BDCPC in SV patients [5, 6, 8, 11, 12], while outcome in relation to pulmonary artery growth has barely been assessed [19].
The aim of this study is to identity patients at risk for PA-stent implantation after BDCPC by evaluating the periinterventional course and assessing the dimensions and growth of the PAs comparing angiographic data pre-BDCPC and pre-total cavopulmonary connection (TCPC) in a group of patients that required PA-stent implantation versus a group that did not require PA-stent implantation.
Methods
Study Design and Patients’ Selection
This is a single center, retrospective, longitudinal analysis of all consecutive SV patients undergoing an invasive hemodynamic and angiographic evaluation pre-BDCPC and pre-TCPC between 2006 and 2021. At least two cardiac catheterizations were performed in each patient (one pre-BDCPC and one pre-TCPC) in accordance with the institutional standards of the University Children’s Hospital Zurich at that time. We excluded all patients with only one cardiac catheterization (prior to BDCPC or prior to TCPC), congenital abnormalities of the pulmonary arteries (specifically, patients with pulmonal atresia and major aortopulmonary collateral arteries after surgical unifocalization), or a pulsatile BDCPC physiology (with a Shunt and a BDCPC) or a delayed BDCPC with a second shunt placement, or patients with a temporary PA-stent (implanted after BDCPC but surgically explanted prior to TCPC).
Demographic data, underlying congenital heart disease, preceding congenital cardiac surgeries or cardiac catheter interventions, indications for cardiac catheterization leading to PA-stent implantation, peri-/post-procedural data, and clinical course until TCPC were analyzed. To evaluate and compare the growth of the pulmonary arteries the Nakata, McGoon, and Total Lower Lobe indices [20, 21]; the LPA and right pulmonary artery (RPA) dimensions normalized for body surface area (BSA); the ratio RPA to LPA and right lower lobe (RLL) to left lower lobe (LLL), and the individual relative growth (%) of each of these parameters were measured angiographically [20, 21]. The measurement sites are shown in Fig. 1. The angiographic dimensions of the (neo-) ascending aorta (at LPA level on a lateral projection) were also assessed and normalized for BSA. Data was analyzed forming two groups: patients undergoing PA-stent implantation after BDCPC (group 1) and patients not undergoing a PA-stent implantation after BDCPC (group 2).
Cardiac Catheterization Procedures
Cardiac catheterizations were performed under general anesthesia. Patients received a 100 IU/kg bolus of heparin intravenously, if they were not anticoagulated prior to cardiac catheterization; otherwise, peri-procedural anticoagulation management was monitored, and titrated measuring activated clotting time. Standard intravenous antibiotic prophylaxis (cephalosporin 2nd generation) was given if a stent was implanted. Biplane angiography was used in all procedures. Anticoagulation continued after catheterization using subcutaneous low-molecular-weight heparin for 24–36 h and was then switched to oral acetylsalicylic acid (3-5 mg/kg/day) monotherapy.
Statistics
Statistical analysis was performed using SPSS 27.0.0 (SPSS Inc, IBM Company, Chicago Illinois/USA). Continuous variables are expressed as median (IQR), categorical data as counts and percentages. Group comparison was performed using two-tailed sample t-tests and Levene’s test for equality of variance was used to analyze, if the variability in the two groups was significantly different. Ordinal, nominal, and dichotomic variables were evaluated with contingency tables and compared with chi-square-tests or Kolmogorov–Smirnov analyses. Predictability of the continuous variables was evaluated by means of Pearson correlations and stepwise multiple regressions, with the criteria probability of F to enter p < 0.05, probability of F to be removed p > 0.1. Binomial logistic regression and Cox stepwise linear regressions were used for dichotomic variables. Significance is defined by values of p < 0.05. A prediction model to estimate the predicted probability of LPA-stent implantation after BDCPC was calculated with the coefficients of the logistic regression model that included the independent predictors of the necessity of LPA-stent implantation after BDCPC. This logistic regression model generated the coefficient of a formula to predict a logit transformation of the probability of LPA-stent implantation after BDCPC. The results of the logit transformation were then converted in predicted probability using the exponential function of the Euler's number. The specificity and sensibility of this model was tested on a ROC-curve.
Ethics
All data were primarily obtained for medical purposes, with informed consent for the performed cardiac catheterization procedure. The study design fulfills the guidelines of the declaration of Helsinki regarding ethical principles for medical research involving human subjects. The study was approved by the institutional ethical board (KEK: 2017–00564).
Results
Patients
A total of 136 patients, 56 (41%) females, were included in the study. A single right ventricle (RV) was present in 69 (51%) of the patients. The complete anatomical characteristics of the patients and surgical/interventional approach at stage I are shown in Table 1.
Peri-and Postinterventional Course
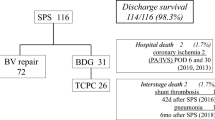
A total of 40/136 (29%) patients underwent PA-stent implantation at median (IQR) 14 (1.1–39) days post-BDCPC at an age of 5 (3–5) months. 37/40 (92.5%) underwent LPA-stenting, 3/40 (7.5%) (2 of which with right aortic arch) RPA-stenting. Reasons for cardiac catheterization were echocardiographically suspected LPA stenosis in 26/40 (65%), RPA stenosis in 1/40 (3%), or low oxygen saturation in 13/40 (33%). Main intraprocedural findings (Fig. 2) at PA-stent implantation after BDCPC were classified as external LPA compression from the ascending aorta (16/40, 40%; in two cases with a complete occlusion of the LPA lumen); LPA hypoplasia (12/40, 30%); LPA stenosis and vascular torsion after surgical anastomosis (11/40, 27.5%); and RPA torsion (1/40, 2.5%). In 7/40 cases (18%) more than one stent was used. No coronary stents were used, and all stents were pre-mounted, balloon expandable, bare metal stents with a closed cell design (e.g., Cordis® Palmaz blue or Palmaz Genesis, or Cook® Formula, or Bentley BeGrow™ stents [22]). Median (IQR) stent diameter was 6 (6–7) mm and length 16 (15–18) mm. Increase of absolute vessel diameter after PA-stent implantation was median (IQR) 3.3 (3.1–4.4) mm and stent-to-stenosis ratio was median 2.4 (IQR 1.9–3.7). Three periinterventional complications occurred: a tracheal bleeding in one patient, and two branch PA dissections requiring surgical intervention. The cause of the dissection was a balloon-dilatation (preceding the stent implantation) across circumferential suture lines in the early postoperative period in the first patient and a dissection after sheath insertion in the second patient. All patients recovered well and periinterventional mortality was zero. Stent re-dilatation was always feasible and performed in 36/40 (89%) after 1 (0.8–1.5) year. Reasons for cardiac catheter reinterventions were stent outgrowth in 26 (72%), neointima formation in 8 (22%), or stent fracture in 2 (6%).
Factors Associated with PA-Stent Implantation
Comparison between group 1 and group 2 showed, that patients with a single RV (p = 0.001), or a hypoplastic left heart syndrome (HLHS)-complex (p < 0.001), or an earlier BDCPC (p = 0.003), or a larger diameter of the (neo-)ascending aorta (normalized for BSA) prior to BDCPC [34.1 (31.1–43.7) versus 31.2 (25.3–35.4) mm/m2; p < 0.0001] had a higher incidence of PA-stent implantation. Patients with no intervention at stage I (p = 0.05) or central pulmonary banding (p = 0.04) at stage I had a lower incidence of PA-stent implantation. All other patients’ characteristics and parameters are shown in Table 1, including the invasive hemodynamics pre-BDCPC, and these were not significantly different between the two groups.
A logistic regression was performed to ascertain the effects of the PA-dimension pre-BDCPC, diameter of the (neo-)ascending aorta pre-BDCPC, and ventricular anatomy on the likelihood of needing a PA-stent implantation after BDCPC. The logistic regression model was statistically significant, χ2(4) = 26.398, p < 0.0001. The model explained 35.0% (Nagelkerke R2) of the variance necessity for PA-stent implantation. Single RV patients were more likely to need a PA-stent than single left ventricle patients (OR 0.41; p = 0.05). Since majority of the single RV patients are HLHS, the increased incidence of LPA stent in these patients can be attributed to a natural “overcorrection” of the neo-aorta often undertaken during surgery. Smaller diameter of LPA (mm/m2) and larger diameter of the (neo-)ascending aorta pre-BDCPC were associated with an increased likelihood of PA-stent implantation after BDCPC (resp. OR 0.89, p = 0.03; OR 1.05, p = 0.001). Age at BDPCP did not add significantly to the model (OR 1.0, p = 0.61).
Based on the results of this logistic regression, we built a prediction model to estimate the predicted probability of LPA-stent implantation after BDCPC.
The equation of the model is (as described in the methods):
We tested the specificity and sensibility of the predicted probability on a ROC-curve (AUC = 0.78) and according to our ROC-curve the threshold with the highest sensitivity and specificity was 0.45 (Fig. 3). This threshold (0.45) correctly classified 65% of the patients with a PA-stent (true positives) and incorrectly included 12% of the patients without a PA-stent (false positive).
Specificity and sensibility of the predicted probability according to our prediction model on a ROC-curve (AUC = 0.78) (A). The threshold with the highest sensitivity and specificity was 0.45. Jittered scatter plot showing how this threshold correctly classified 65% of the patients with a PA-stent (true positives) and incorrectly included 12% of the patients without a PA-stent (false positive) (B)
Pulmonary Artery Dimensions and Growth (Table 2 and Fig. 4 )
Collective pulmonary artery diameters pre-BDCPC were lower in the PA-stent group: Nakata (p < 0.001), RPA mm/m2 (p = 0.01), and LPA mm/m2 (p = 0.001). Lower Lobe Index was lower, but not significantly (p = 0.08). While absolute pulmonary arteries diameters increased in both groups, indexed pulmonary artery diameters decreased equally in both groups, but remained lower pre-TCPC in the PA-stent group: Nakata (p = 0.009), RPA mm/m2 (p = 0.01), and LPA mm/m2 (p = 0.01), Lower Lobe Index (p = 0.003). LPA and RPA grew symmetrically in both groups
At cardiac catheterization pre-BDCP the PA-stent group was younger (p = 0.007). Overall, pulmonary artery diameters pre-BDCPC were lower in the PA-stent group, specifically Nakata (p < 0.001), RPA mm/m2 (p = 0.01), and LPA mm/m2 (p = 0.001). Lower Lobe Index was tendentially lower, however not reaching statistical significance (p = 0.08). From the assessment pre-BDCPC and pre-TCPC absolute pulmonary arteries diameters increased in both groups but indexed pulmonary artery diameters decreased in both groups. The individual relative growth (%) was not different within the groups for all parameters. At cardiac catheterization pre-TCPC age was similar between the groups. Nakata (p = 0.009), RPA mm/m2 (p = 0.01), and LPA mm/m2 (p = 0.01), and Lower Lobe Index (p = 0.003) remained lower pre-TCPC in the PA-stent group. RPA:LPA ratio and LLL:RLL ratio remained symmetric at both assessments between and within the groups.
Discussion
This investigation is one of the first studies analyzing pulmonary artery growth pattern between the time of BDCPC and TCPC focusing on the potential beneficial impact of central pulmonary artery stenting [7]. Our data show that PA stenting becomes frequently necessary, as shown by others [5,6,7,8,9,10], and may ensure symmetric PA growth comparable with the non-stented PA side branch.
Factors associated with need for central PA stenting may be predicted before BDCPC, especially for the LPA, which is predominantly affected. This includes smaller indexed diameter of LPA pre-BDCPC, larger diameter of the (neo-) ascending aorta pre-BDCPC, and cardiac anatomy consistent with HLHS-complex. These predictors align with the intraprocedural findings, according to which a compression from the (neo-) ascending aorta presumably is the main cause of LPA stenosis, whereas two of three patients that underwent RPA-stenting had a right aortic arch.
Pulsatile compression from a larger (neo-) ascending aorta is an important anatomic feature for potential postoperative LPA compression [7, 8, 11, 12]. Especially in HLHS-complex patients the position of the Damus-Kaye-Stansel anastomosis moves the ascending aorta more to the left in comparison to patients with a native aortic arch, while the reconstruction of the inner curvature of the aortic arch with xenopericardium may lead to foreshortening over time and thus decreases the space under the arch through which LPA and left bronchus pass. These could contribute to LPA—and bronchus—compression [1].
The surgical approach with a comprehensive stage I and II had been associated with higher incidence of PA-interventions [8, 9], but this was not a significant risk in our cohort. Further on, earlier BDCPC seems to affect only marginally PA growth. All other assessed anatomical and surgical factors are not associated with an increased risk for PA-stent implantation after BDCPC.
SV patients, depending on their cardiac anatomy, might have small pulmonary arteries directly after birth [2,3,4]. During the interval between stage I and II they can have a “catch-up” growth due to pulmonary overflow and pulsatile pulmonary blood flow (Sano shunt), while after stage II they normally have an inadequate development of the pulmonary vasculature, that could lead to increased pulmonary vascular resistance [2,3,4]. BDCPC creation from a shunted single ventricle leads to volume unloading, which may lead to under-filling of the branch PAs. These considerations are in line with the relative decrease of the size of PAs between BDCPC and TCPC found in our analysis and previous assessments in literature [23,24,25].
Since the growth of the PAs depends on blood flow [26], improvement in the caliber of the LPA by PA-stent implantation could potentially maximize future growth [7]. Currently, there is limited data published on distal PA growth after PA-stent implantation. One single analysis [19] on 18 SV patients showed, a similar growth of the stented and non-stented contralateral pulmonary artery branches, which is in line with our findings. In our cohort, we could also show that the growth of the PA-system is not only symmetric with the contralateral side, but also similar to the group without a PA-stent. As expected, a PA-stent implantation does not help to fully “catch-up” in growth, but ensures a PA growth comparable to non-PA stented patients [2,3,4]. However, other studies have shown direct positive implications of a LPA-stent implantation. In fact, an increase in cardiac output associated with LPA-stenting in SV patients has been demonstrated [27], while MRI-based studies have shown, that zones of narrowing within the non-pulsatile pulmonary artery circuit relate to relevant energy losses in proportion to the degree of narrowing [1].
It is well reported in literature, that early postoperative cardiac catheterization after surgery for congenital heart disease is feasible and safe, even when acting on freshly formed sutures or hemodynamic instable patients [5, 12, 14,15,16,17]. Stent implantation to treat PA stenosis in SV patients is effective and can be realized safely [7, 10]. In other cohorts [15, 27, 28], angioplasty was performed safely on fresh suture lines without reported vascular tear or suture disruption. In our cohort, one patient probably experienced a suture disruption, but this was related to the balloon-angioplasty prior to LPA-stent implantation. This is concordant with reports from Zahn et al. [29], which concluded that continuous Prolene suture lines can be expanded safely without disruption when using balloon to stenosis ratio ≤ 2.5/1 [29]. In our cohort, we used a stent-to-stenosis ratio of 2.4 (IQR 1.9–3.7). Nevertheless, a not negligible periprocedural risk profile of PA stenting early after BDCPC remains and an immediate surgical backup dealing with severe PA bleeding complications is necessary [17].
The selection of a particular stent is mainly depended on the size of the reference vessels [29, 30], the characteristics of the stenosis [13], and the size of the patient with regards to the sheath size needed for implantation. The initial diameter of the stent was chosen conservatively without overdilating the stenosed area. Since the rate of vessel complications associated with PA-stenting is low, bare metal (instead of covered) stents were used.
Since stents implantation was performed in growing vessels, repetitive stent re-dilatations were performed to accommodate the vessel diameter for somatic growth. Stent re-dilatation was always feasible [31].
When performing stent implantation stents that can be dilatated to reach the adult diameter of the pulmonary arteries (ca. 15 mm) should be preferred [32]. Until now, all available stents have limited final expansion diameters. Breakable stents, such as the Bentley BeGrow™ stents, could be a viable option in some cases since they can be implanted in small vessels (4-6 mm) with a 4 French sheath but have the potential for further re-dilations and controlled stent strut breakage to fit with the growth of the contralateral pulmonary branch [22]. Bioabsorbable stents could also potentially allow further growth of the artery and remodeling of the vessel wall [33].
A model for prediction of the necessity for an LPA-stent implantation after BDCPC would be an appealing tool for the clinician and could potentially lead to a change in the clinical management of these patients. In fact, our prediction model might estimate the probability of the necessity for PA-stent implantation and could be used to (a) perform a close follow-up or (b) plan an exit-angiography directly after BDCPC in patients at risk for pulmonary artery complications or, in high-risk patients, (c) perform a hybrid procedure for LPA stenting during BDCPC surgery.
In summary, a stent implantation (most often for external LPA compression), when clinically indicated, ensures symmetrical pulmonary flow and growth of the hilar and intrapulmonary vessels. However, side branch PA stents require multiple catheter interventions to match the child’s growth.
Limitation
Our study is a retrospective study with its inherent limitations. A group comparison with patients that had a stenosis in the PA-system but did not receive a PA-stent is impossible from an ethical perspective. The growth of the pulmonary arteries is influenced by many other factors, such as the blood flow distribution to the left and right PA, that we did not assess. The direct clinical impact of the PA-implantation is hard to define and therefore not assessed in this analysis. The aim of our study focused on the time frame between BDCPC and TCPC rather than long-term outcome. Further surgical factors such as placement of the pulmonary artery confluence to the left or right of the midline [1, 34] could not been evaluated. The architecture of the aortic arch other than the dimensions of the ascending aorta has not been evaluated (34). Lastly, our prediction model is based on the results of our institution, and it might not fit for another institution with a different surgical or interventional strategy.
Conclusion
PA-stent implantation early after BDCPC is feasible and safe. Single RV, larger (neo-) ascending aorta, and smaller LPA prior to BDCPC are independent risk factors for PA-stent implantation early post-BDCPC. Our results suggest a pre-BDCPC risk-stratification for PA-stent implantation is theoretically feasible and at least an exit-angiography should be discussed in risk cases. PA diameters after PA-stent implantation and stent dilatation show symmetric but limited growth together with the contralateral side, similar to the no-PA-stent group. Therefore, PA-stents do not negatively influence peripheral PAs growth and should not be withheld, if clinically indicated.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article. Supplementary data that support the findings of this study are available from the corresponding author, AC, upon reasonable request.
References
Krimly A, Jain CC, Egbe A, Alzahrani A, Najashi KA, Albert-Brotons D et al (2021) The pulmonary vascular bed in patients with functionally univentricular physiology and a Fontan circulation. Cardiol Young 31(8):1241–1250
Gewillig M, Brown SC (2016) The Fontan circulation after 45 years: update in physiology. Heart 102(14):1081–6
Gewillig M, Brown SC, Eyskens B, Heying R, Ganame J, Budts W et al (2010) The Fontan circulation: who controls cardiac output? Interact Cardiovasc Thorac Surg 10(3):428–433
Gewillig M, Boshoff DE. Missing a Sub-pulmonary Ventricle: The Fontan Circulation. In: Voelkel NF, Schranz D, editors. The Right Ventricle in Health and Disease [Internet]. New York, NY: Springer New York; 2015 [cited 2022 Feb 6]. p. 135–57. (Respiratory Medicine). Available from: http://link.springer.com/https://doi.org/10.1007/978-1-4939-1065-6_8
Kojima T, Imamura T, Osada Y, Muraji S, Nakano M, Oyanagi T et al (2018) Efficacy of catheter interventions in the early and very early postoperative period after CHD operation. Cardiol Young 28(12):1426–1430
Kretschmar O, Sglimbea A, Prêtre R, Knirsch W (2009) Pulmonary artery stent implantation in children with single ventricle malformation before and after completion of partial and total cavopulmonary connections. J Interv Cardiol 22(3):285–290
Noonan P, Kudumula V, Anderson B, Ramchandani B, Miller P, Dhillon R et al (2016) Stenting of the left pulmonary artery after palliation of hypoplastic left heart syndrome. Catheter Cardiovasc Interv 88(2):225–232
Dave H, Rosser B, Knirsch W, Hubler M, Pretre R, Kretschmar O (2014) Hybrid approach for hypoplastic left heart syndrome and its variants: the fate of the pulmonary arteries. Eur J Cardiothorac Surg 46(1):14–19
Rahkonen O, Chaturvedi RR, Benson L, Honjo O, Caldarone CA, Lee KJ (2015) Pulmonary artery stenosis in hybrid single-ventricle palliation: High incidence of left pulmonary artery intervention. J Thorac Cardiovasc Surg 149(4):1102-1110.e2
Moszura T, Dryzek P, Goreczny S, Mazurek-Kula A, Moll JJ, Sysa A et al (2014) A 10-year single-centre experience in percutaneous interventions for multi-stage treatment of hypoplastic left heart syndrome. Cardiol Young 24(1):54–63
Fujii T, Tomita H, Otsuki S, Kobayashi T, Ono Y, Yazaki S et al (2014) Stenting for pulmonary artery stenosis complicated by univentricular physiology: subanalysis of JPIC stent survey. J Cardiol 64(4):324–327
Bhole V, Wright JGC, De Giovanni JV, Dhillon R, Miller PA, Desai T et al (2011) Transcatheter interventions in the early postoperative period after the Fontan procedure. Catheter Cardiovasc Interv 77(1):92–98
Khan A, Ing FF (2013) Catheter interventions for pulmonary artery stenosis: matching the intervention with the pathology. Interv Cardiol Clin 2(1):131–151
Eraso-Díaz Del Castillo AM, Escobar-Díaz MC, Lince Varela R, Díaz Medina LH, Cañas Arenas EM (2019) Catheterization performed in the early postoperative period after congenital heart surgery in children. Pediatr Cardiol 40(4):827–833
Bahaidarah S, Al-Ata J, Abdelmohsen G, Alkhushi N, Abdelsalam M, Mujahed M, et al. Cardiac catheterization addressing early post-operative complications in congenital heart surgery—a single-center experience. Egypt Heart J [Internet]. 2020 Nov 23 [cited 2021 Feb 1];72. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7683629/
Quandt D, Callegari A, Niesse O, Christmann M, Meinhold A, Dave H, et al. Early Cardiac Catheterizations within 30 Days Post Congenital Heart Surgery in Children. Congenital Heart Disease. 2022;0(0):1–17.
Quandt D, Callegari A, Niesse O, Meinhold A, Dave H, Knirsch W et al (2022) Balloon angioplasty and stent implantation within 30 days postcongenital heart surgery (CHS) in children. J Card Surg. https://doi.org/10.1111/jocs.17057
Mets JM, Bergersen L, Mayer JE, Marshall AC, McElhinney DB (2013) Outcomes of stent implantation for obstruction of intracardiac lateral tunnel Fontan pathways. Circ Cardiovasc Interv 6(1):92–100
Takao CM, El Said H, Connolly D, Hamzeh RK, Ing FF (2013) Impact of stent implantation on pulmonary artery growth. Catheter Cardiovasc Interv 82(3):445–452
Nakata S, Imai Y, Takanashi Y, Kurosawa H, Tezuka K, Nakazawa M et al (1984) A new method for the quantitative standardization of cross-sectional areas of the pulmonary arteries in congenital heart diseases with decreased pulmonary blood flow. J Thorac Cardiovasc Surg 88(4):610–619
McGoon DC, Baird DK, Davis GD (1975) Surgical management of large bronchial collateral arteries with pulmonary stenosis or atresia. Circulation 52(1):109–118
Quandt D, Knirsch W, Michel-Behnke I, Kitzmüller E, Obradovic M, Uhlemann F et al (2019) First-in-man pulmonary artery stenting in children using the Bentley® BeGrow™ stent system for newborns and infants. Int J Cardiol 1(276):107–109
Kansy A, Brzezińska-Rajszys G, Zubrzycka M, Mirkowicz-Małek M, Maruszewski P, Manowska M et al (2013) Pulmonary artery growth in univentricular physiology patients. Kardiol Pol (Polish Heart Journal) 71(6):581–587
Mendelsohn AM, Bove EL, Lupinetti FM, Crowley DC, Lloyd TR, Beekman RH (1994) Central pulmonary artery growth patterns after the bidirectional Glenn procedure. J Thorac Cardiovasc Surg 107(5):1284–1290
Slavik Z, Franklin RC, Radley-Smith R (1999) The real fate of pulmonary arteries after bidirectional superior cavopulmonary anastomosis: is there a need for concern? Cardiol Young 9(1):6–10
Sievers HH, Onnasch DG, Lange PE, Bernhard A, Heintzen PH (1983) Dimensions of the great arteries, semilunar valve roots, and right ventricular outflow tract during growth: normative angiocardiographic data. Pediatr Cardiol 4(3):189–196
Noonan PME, Viswanathan S, Chambers A, Stumper O (2014) Non-invasive cardiac output monitoring during catheter interventions in patients with cavopulmonary circulations. Cardiol Young 24(3):417–421
Downing TE, Allen KY, Goldberg DJ, Rogers LS, Ravishankar C, Rychik J et al (2017) Surgical and catheter-based reinterventions are common in long-term survivors of the Fontan operation. Circ: Cardiovasc Interv 10(9):e004924
Zahn EM, Dobrolet NC, Nykanen DG, Ojito J, Hannan RL, Burke RP (2004) Interventional catheterization performed in the early postoperative period after congenital heart surgery in children. J Am Coll Cardiol 43(7):1264–1269
Nicholson GT, Kim DW, Vincent RN, Petit CJ (2015) Transcatheter interventions across fresh suture lines in infants and children: an 8-year experience. Catheter Cardiovasc Interv 86(2):271–277
McMahon CJ, El-Said HG, Grifka RG, Fraley JK, Nihill MR, Mullins CE (2001) Redilation of endovascular stents in congenital heart disease: factors implicated in the development of restenosis and neointimal proliferation. J Am Coll Cardiol 38(2):521–526
Ovroutski S, Ewert P, Alexi-Meskishvili V, Hölscher K, Miera O, Peters B et al (2009) Absence of pulmonary artery growth after Fontan operation and its possible impact on late outcome. Ann Thorac Surg 87(3):826–831
Zartner P, Cesnjevar R, Singer H, Weyand M (2005) First successful implantation of a biodegradable metal stent into the left pulmonary artery of a preterm baby. Catheter Cardiovasc Interv 66(4):590–594
Nassar MS, Bertaud S, Goreczny S, Greil G, Austin CB, Salih C et al (2015) Technical and anatomical factors affecting the size of the branch pulmonary arteries following first-stage Norwood palliation for hypoplastic left heart syndrome. Interact CardioVasc Thorac Surg 20(5):631–635
Funding
Open access funding provided by University of Zurich. All authors have no potential conflicts of funding or financial relationship to disclose.
Author information
Authors and Affiliations
Contributions
AC, JL, WK, RC, HD, OK, DQ all authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no potential conflict of interest to disclose.
Ethical Approval
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Callegari, A., Logoteta, J., Knirsch, W. et al. Risk Factors and Outcome of Pulmonary Artery Stenting After Bidirectional Cavopulmonary Connection (BDCPC) in Single Ventricle Circulation. Pediatr Cardiol 44, 1495–1505 (2023). https://doi.org/10.1007/s00246-023-03229-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-023-03229-3