Abstract
Transcatheter closure of Perimembranous VSDs (PMVSD) remains challenging particularly in infants. The aim of this study is to evaluate the efficacy and safety of transfemoral PMVSD device closure in infants weighing ≤ 10 kg in a single centre. Retrospective review of departmental databases and medical charts to define patient cohort and collect demographic, procedural and follow-up data. Between July 2014 and March 2021, 16 patients underwent attempted transfemoral PMVSD device closure (12 retrograde) at a median age of 11 months (interquartile range [IQR] 9–15.5) and a median weight of 8.3 kg (IQR 7.2–9.5). All patients were either symptomatic, had progressive left heart dilation or had VSD associated valve regurgitation. Median defect size on pre-procedural transoesophageal echocardiography was 6.8 mm (IQR 6–8.5). Median device waist size was 6 mm (IQR 4.5–8). Successful device placement was achieved in 14 patients (88%). One patient developed moderate aortic and tricuspid valve regurgitation upon retrograde and antegrade device deployment, respectively, and subsequently underwent surgical closure. The second patient developed progressive aortic regurgitation (AR) 2 days post procedure, and also underwent surgical removal with no residual AR. There was no cases of device embolization and no femoral arterial compromise. On median follow-up of 40.5 months (IQR 25–64), none of the patients developed complete heart block. Three patients (18.75%) had small residual shunts at latest follow-up which have not required any further intervention. Device closure of PMVSD’s in children weighing ≤ 10 kg is feasible and safe with good procedural success rates. Use of both the antegrade and retrograde approaches may be necessary depending on anatomical variances.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgical closure remains the standard approach for perimembranous VSD (PMVSD) closure especially in infants. However, there is a relatively high morbidity as patients require intensive care unit (ICU) admission and often require blood transfusions [1]. These smaller infants also have a higher risk of AV block [1]. In relation to longer-term consequences, a large population follow-up study has suggested surgical closure is associated with a hazard ratio of 13 compared to the general population of developing atrial fibrillation in the third decade of life [2].
Transcatheter device closure of PMVSDs avoids cardiopulmonary bypass and is associated with shorter hospital stay and less morbidity. Initial concerns regarding unacceptable rates of complete heart block with first generation devices appear to have been circumvented with newer device design, suggesting softer devices with less radial forces might exert less compression on the conduction system [3,4,5,6,7,8,9,10]. Indeed, a recent meta-analysis of transcatheter PMVSD closure in over 6,300 patients revealed a complete atrio-ventricular block (cAVB) rate comparable to surgery at 1.1% [11].
Percutaneous closure of PMVSD in children weighing ≤ 10 kg has been reported to be associated with procedural failure, procedure- or device related adverse events with longer fluoroscopy times [12]. It is technically a greater challenge in lower weight patients with smaller femoral vessels and creation of arteriovenous loops in these patients may result in rhythm disturbances and haemodynamic compromise [13].
Few studies dealing with device closure of PMVSD’s have been published on this subgroup of patients with varying approaches including a trans-carotid approach reported to mitigate against the possible hemodynamic impact of an arteriovenous loop [13,14,15]. The aim of this study was to evaluate the efficacy and safety of transfemoral PMVSD device closure in infants ≤ 10 kg body weight in a national tertiary care congenital cardiac institution.
Methodology
Retrospective review of all patients ≤ 10 kgs who underwent attempted closure of PMVSD via a transfemoral approach. Patients were recruited from a single tertiary cardiology centre (Children’s Health Ireland at Crumlin, Dublin, Ireland). Patients were identified and data collected from the National Institute of Cardiology Outcomes Research (NICOR) database to obtain demographic and procedural details. Institutional review board approval was obtained by the Hospital’s Research Ethics board. All procedures which were performed over a 7-year period between July 2014 and March 2021 were included.
Patient Selection
The decision to utilize a transfemoral approach for VSD closure in each patient was made following multi-disciplinary discussion at the joint cardiology/cardiothoracic conference.
Patients were included if they had a hemodynamically significant (symptomatic and/or left ventricular dilation, significant LV-RA shunt or progressive aortic regurgitation) PMVSD; weight ≤ 10 kg at time of procedure and the procedure was attempted via a transfemoral approach.
Patients were excluded if they had more than mild aortic regurgitation (AR), significant associated congenital heart disease, significant septal malalignment or aortic leaflet prolapse.
Demographic Details
Data collected included demographic details (patient age, gender, weight, height, and body surface area at the time of the procedure); echocardiographic data (VSD measurement [LV entry, RV exit, VSD rims, presence or absence of aneurysmal tissue], associated anomalies, aortic, mitral or tricuspid valve regurgitation); and procedural data (sheath size, access type, angiographic VSD size, number of devices, associated procedures, residual shunting, fluoroscopic, procedural times, rhythm changes and reasons for failure if applicable).
Procedural success was defined by device implantation in the appropriate position without conversion to open heart surgery; which is our primary outcome. Secondary outcomes included any significant adverse events in relation to the procedure including death, rhythm disturbance, device migration, significant residual shunting (> 2 mm), requirement for pacemaker, or vascular access-related complication.
Procedural Technique
Initially, transoesophageal echocardiogram (TOE) was performed under general anaesthesia to assess defect size, LV entry, RV exit, VSD rims, presence or absence of aneurysmal tissue and degree of valvular regurgitation, in order to determine suitability for device closure, and to aid in device selection (Fig. 1).
Patients were covered with periprocedural prophylactic antibiotics. Femoral arterial and venous access was obtained predominantly under ultrasound guidance after which 100 units/kg of IV Heparin was administered.
Left ventricular (LV) angiography was performed in a left anterior oblique view (Fig. 2).
The approach and the type of device was determined based on presence of a ventricular septal aneurysm, length of subaortic rim and VSD diameter with the retrograde (from the aorta) approach often initially preferred. The selected VSD device was chosen to be 1–2 mm greater than the largest TOE measurement of the VSD with colour flow. Once deployed, careful assessment of the interaction with the aortic and tricuspid valves was carried out with TOE. Cable induced aortic regurgitation and the inability to perform pre-release LV angiography have led to greater dependence on TOE imaging.
Post-procedure two additional doses of antibiotics were given every 8 h in the first 24 h. Aspirin was commenced at 5 mg/kg for 6 months. A comprehensive transthoracic echocardiogram and 12-lead ECG were performed on the day following the procedure.
Statistical Analysis
Data were summarized as follows: continuous variables were summarized as median and interquartile range (IQR). Categorical data were summarized as count. Analyses were performed using Excel statistical function (Microsoft office standard 2013).
Results
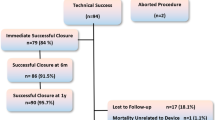
Sixteen patients underwent attempted transfemoral PMVSD device closure (12 retrograde) at a median age of 11 months (interquartile range [IQR] 9–15.5) and a median weight of 8.3 kg (IQR 7.2–9.5). Most patients were either symptomatic or had progressive left heart dilation. One patient had a significant LV-RA shunt with 3 + TR. Another patient had mild AR without aortic leaflet prolapse thought be related to the VSD. Median defect size on pre-procedural TOE was 6.8 mm (IQR 6–8.5). Median device waist size was 6 mm (IQR 4.5–8).
Genetic abnormalities were present in three patients, two of whom had 22q11 microdeletion and one with Down syndrome (Table 1).
Procedural Outcomes
Six patients received Amplatzer Duct Occluder II (Abbott, Clonmel, Tipperary, Ireland); 4 patients received Lifetech Symmetric Membranous VSD Occluder (Lifetech, Shenzhen, China); 4 patients received KONAR-MF VSD Occluder (Lifetech, Shenzhen, China); 1 patient received Lifetech Eccentric Membranous VSD Occluder (Lifetech, Shenzhen, China); and 1 patient received Occlutech PmVSD Occluder (Occlutech, Helsingborg, Sweden). Median procedural and fluoroscopy times were 81 min and 12 min, respectively.
Median (IQR) device waist size was 6.2 mm (5.7–8.3 mm) (Table 2). Successful device placement was achieved in 14 patients (88%). Two patients required two devices due to significant residual shunt after release of the first device into a fenestrated defect (retrograde approach in both). Of the two unsuccessful device placements, one patient developed moderate aortic valve regurgitation (AR) and tricuspid valve regurgitation (TR) upon retrograde and antegrade device deployment, respectively. The device was not released and the patient was subsequently sent for elective surgical closure. The other patient developed progressive moderate AR 2 days post procedure, and the patient underwent surgical removal with no residual AR. One further patient developed transient haemolysis which resolved spontaneously within 1 week. There were no cases of device embolization and no other procedural complications, including no femoral arterial compromise. None of the patients required emergency surgery.
Median hospital stay was 1 day (IQR 1–1). On median follow-up of 40.5 months (IQR 25–64), none of the patients developed complete heart block. Three patients (18.75%) had small residual shunts at latest follow-up which have not required any further intervention.
Discussion
Since the initial report of transcatheter VSD closure by Lock et al. [16], the advent of transcatheter closure has progressed rapidly, initially with muscular VSD closure [17,18,19] and subsequently with PMVSD closure [20]. Early large series reported the challenges faced when dealing with infants weighing less than 10 kgs [13]. With this single centre experience, we report good success rates (88%) with device closure of PMVSD’s in this challenging cohort of patients with a median weight of 8kgs and defect size of 6 mm.
Often, the most challenging aspect of these cases in these smaller patients is pre-determination of device interaction with the tricuspid or aortic valves prior to release. This is particularly challenging in PMVSD’s where the aortic rim is deficient leading to potential impingement on the aortic valve resulting in significant aortic regurgitation [21,22,23]. Long-term rates of aortic regurgitation (AR) are unclear however a recent presentation evaluating 10-year follow-up with the original Amplatzer membranous occluder in 95 patients, demonstrated mild stable AR in 3 patients [24]. Significant aortic regurgitation requiring surgical removal has been reported at 1.8% [25]. AR may also occur in the absence of leaflet impingement and some authors have suggested distortion of the aortic annulus or subaortic membrane configuration with larger devices or by repeated crossing of the aortic valve [26]. It has also been suggested that when trivial or mild, the degree of AR many remain stable or decrease over time [26]. The improvement of AR is thought to be likely secondary to normalization of valve configuration changes created by repeated valve crossing or by change in configuration of the thin-walled membranous septum which improves with time [26]. It may be challenging to distinguish leaflet impingement from cable induced aortic distortion when performing these cases via the retrograde arterial approach and careful echocardiographic evaluation is essential to differentiate these two possibilities.
In this series the aortic valve was assessed closely with echocardiography upon crossing the VSD with the sheath, before device deployment and then before device release. Furthermore, ascending aortography was sometimes performed to complement echocardiographic images and delineate the extent, if any, of the AR. Although interpretation of aortography with retrograde delivery was less helpful, when performed through the side arm of the delivery sheath, this usually provided further information regarding potential device interaction with the valve leaflets. Multiple evaluations with echocardiography at different time points are important to understand the exact mechanism of AR and comparative evaluations once the sheath has been advanced into the RV and following device deployment can be useful in differentiating delivery cable from device related regurgitation.
Despite detailed assessment with complimentary imaging, two patients developed AR. The first patient developed moderate AR upon retrograde device deployment. The pre-procedural echocardiogram demonstrated an associated septal aneurysm, which has been shown to reduce the risk of AR [13]. On repositioning of the device using the antegrade approach the patient subsequently developed significant tricuspid regurgitation (TR). The device was recaptured and the patient was referred for surgery. In the second patient, who was noted to have a small aortic rim measuring < 2 mm, the device was deployed successfully initially with minimal AR. However, the patient developed progressive AR 2 days post procedure, and subsequently underwent surgical removal with no residual AR. Surgical examination intra-operatively revealed an intact aortic valve with no signs of aortic leaflet damage These two cases demonstrate the importance of a thorough assessment not only prior and during device deployment, but also in the early post-procedural period. They also highlight the importance of outlining the risk of valve distortion when consenting patients or their guardians.
Fourteen of the devices were implanted via the retrograde approach. In comparison to the antegrade approach, this approach involves comparatively fewer steps and carries the potential to significantly reduce fluoroscopy and total procedural times [27]. Avoidance of arteriovenous looping may also avoid cardiovascular compromise created by splinting of the heart. As has been reported in previous series, the avoidance of the arteriovenous loop is particularly important in small hearts as the wire and sheath crossing the tricuspid and aortic valves may cause severe haemodynamic compromise [23].
As most of the patients had an aneurysmal pouch, all but 1 patient underwent symmetric double-disc occluders. Only one eccentric membranous occluder was used in a patient where the aortic rim was incomplete.
There have been concerns about large sheaths and delivery systems being used in the femoral arteries of these small children. However, the relatively large delivery systems used in the femoral artery in this series were well tolerated and none of the patients who underwent retrograde closure suffered femoral arterial compromise. Occasionally, modification of the delivery cable is required to minimize traction on the deployed LV disc caused by a straight delivery cable around the relatively acute natural curves of the aortic arch in a small heart (Fig. 3).
One of the less well considered benefits of the retrograde approach is the avoidance of delivery sheath disruption of the tricuspid valve (TV). However, it is important when using the retrograde approach to consider deploying the TV disc only when the support mechanism of the TV has been cleared, usually best seen using live TOE.
Mechanisms leading to tricuspid regurgitation (TR) with PMVSD closure include chordae tendinea injury or improper placement or oversizing of the device [28]. The exact anatomical variations in the relationship of the TV to the VSD that can predict procedural failure due to new onset TR have been challenging to identify. Certainly, cases where the defect extends up to the base of the septal leaflet of the TV may be more susceptible to device related distortion of the septal leaflet. This is likely to be relevant for those devices with larger “RV” discs including the ADO II. The incidence of TR with the ADO II is estimated to be 0.4 to 8% [26], 29,30,31,32. As the device is not particularly rigid the degree of TR in older patients is mostly mild and improves with time [26, 31]. Pre-existing TV regurgitation, which may be considered another indication to close the VSD, is reported to improve with PMVSD device closure. The mechanism is likely to be related to the commonly associated LV-RA shunt [26, 31, 33, 34]. One of our patients had pre-existing moderate to severe tricuspid regurgitation that decreased to mild degree after device closure.
While we favour the retrograde approach and it has the aforementioned benefits, flexibility during the case is important. While using the antegrade approach, it’s important to cross the TV using a balloon tipped catheter thus reducing the risk of TV chordae injury. The relationship between any aneurysmal tissue and tricuspid valve leaflet is an important consideration as a TV with a redundant leaflet may appear to be part of an aneurysm i.e. pseudoaneurysm but this is not a distinct true aneurysm leading to the device disc clamping on the TV chordae [31, 35].
Narin et al. reported transcatheter VSD closure in 12 patients less than 1 year of age via a transfemoral approach [36]. Eight were PMVSDs and 6 of these were performed via an antegrade approach. The smallest patient’s weight in their group was 4.8 kg. The choice of approach is largely dependent on the presence or absence of adequate aortic rim, presence of a VSD aneurysm and operator experience. In our centre, the initial attempt is generally via a retrograde approach, especially in the presence of a sufficient aortic rim and/or VSD aneurysm, with flexibility to revert to the transvenous approach if necessary.
While valvular regurgitation is a concern with transcatheter PMVSD closure, atrio-ventricular (AV) block has historically been the most feared complication [11]. In our series no patient in the follow-up period developed either transient or permanent AV block. A large meta-analysis has reported a rate of complete AV block of 1.1% with very few patients requiring permanent pacemaker insertion [11]. This was a heterogenous group of studies, and the age and weight of patients were not considered due to inconsistent reporting in the evaluated studies. Similar to this meta-analysis, we demonstrated excellent results when considering potential AV block complications.
Conclusion
This series demonstrates that device closure of PMVSD’s in children weighing ≤ 10 kg is feasible and safe with good procedural success rates. Understanding the importance of complimentary imaging along with flexibility with approach ensures success with limited complications. Ultimately longer-term follow-up data are required as concerns still exist in relation to the possible interaction of the device in particularly with the aortic valve.
Data Availability
Data available on request from the authors.
References
Ergün S, Genç SB, Yildiz O, Öztürk E, Kafalı HC, Ayyıldız P et al (2019) Risk factors for major adverse events after surgical closure of ventricular septal defect in patients less than 1 year of age: a single-center retrospective. Braz J Cardiovasc Surg 34(3):335–343
Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Gilljam T, Hansson PO et al (2018) Atrial fibrillation burden in young patients with congenital heart disease. Circulation 137(9):928–937
Vijayalakshmi IB, Narasimhan C, Singh B, Manjunath CN (2017) Treatment of congenital non-ductal shunt lesions with the amplatzer duct occluder II. Catheter Cardiovasc Interv 89(6):E185–E193
Bergmann M, Germann CP, Nordmeyer J, Peters B, Berger F, Schubert S (2021) Short- and long-term outcome after interventional VSD closure: a single-center experience in pediatric and adult patients. Pediatr Cardiol 42(1):78–88
Nguyen HL, Phan QT, Dinh LH, Tran HB, Won H, Thottian JJ et al (2018) Nit-Occlud Lê VSD coil versus Duct Occluders for percutaneous perimembranous ventricular septal defect closure. Congenit Heart Dis 13(4):584–593
El Shedoudy S, El-Doklah E (2019) Mid-term results of transcatheter closure of ventricular septal defect using Nit-Occlud Lê ventricular septal defect coil, single-center experience. J Saudi Heart Assoc 31(2):78–87
Andersen HØ, de Leval MR, Tsang VT, Elliott MJ, Anderson RH, Cook AC (2006) Is complete heart block after surgical closure of ventricular septum defects still an issue? Ann Thorac Surg 82(3):948–956
Edwin F, Aniteye E, Tettey M, Sereboe L, Kotei D, Tamatey M et al (2010) Permanent complete heart block following surgical correction of congenital heart disease. Ghana Med J 44(3):109–114
Ghaderian M, Merajie M, Mortezaeian H, Aarabi M, Mohammad Y, Shah MA (2015) Efficacy and safety of using amplatzer ductal occluder for transcatheter closure of perimembranous ventricular septal defect in pediatrics. Iran J Pediatr 25(2):e386
Siehr SL, Hanley FL, Reddy VM, Miyake CY, Dubin AM (2014) Incidence and risk factors of complete atrioventricular block after operative ventricular septal defect repair. Congenit Heart Dis 9(3):211–215
Santhanam H, Yang L, Chen Z, Tai BC, Rajgor DD, Quek SC (2018) A meta-analysis of transcatheter device closure of perimembranous ventricular septal defect. Int J Cardiol 254:75–83
Holzer R, de Giovanni J, Walsh KP, Tometzki A, Goh T, Hakim F et al (2006) Transcatheter closure of perimembranous ventricular septal defects using the amplatzer membranous VSD occluder: immediate and midterm results of an international registry. Catheter Cardiovasc Interv 68(4):620–628
Diab KA, Cao QL, Mora BN, Hijazi ZM (2007) Device closure of muscular ventricular septal defects in infants less than one year of age using the Amplatzer devices: feasibility and outcome. Catheter Cardiovasc Interv 70(1):90–97
Pillai AA, Rangasamy S, Balasubramonian VR (2019) Transcatheter closure of moderate to large perimembranous ventricular septal defects in children weighing 10 kilograms or less. World J Pediatr Congenit Heart Surg 10(3):278–285
Breatnach CR, Kenny D, Linnane N, Al Nasef M, Ng LY, McGuinness J et al (2021) Transcarotid approach to ventricular septal defect closure in small infants. Pediatr Cardiol 42(7):1539–1545
Lock JE, Block PC, McKay RG, Baim DS, Keane JF (1988) Transcatheter closure of ventricular septal defects. Circulation 78(2):361–368
Hijazi ZM, Hakim F, Al-Fadley F, Abdelhamid J, Cao QL (2000) Transcatheter closure of single muscular ventricular septal defects using the Amplatzer muscular VSD occluder: initial results and technical considerations. Catheter Cardiovasc Interv 49(2):167–172
Thanopoulos BD, Tsaousis GS, Konstadopoulou GN, Zarayelyan AG (1999) Transcatheter closure of muscular ventricular septal defects with the amplatzer ventricular septal defect occluder: initial clinical applications in children. The Amplatzer VSD occluders were provided by AGA, Medical Corporation, Golden Valley, Minnesota. There were no sources of financial support. J Am Coll Cardiol 33(5):1395–1399
Waight DJ, Bacha EA, Kahana M, Cao QL, Heitschmidt M, Hijazi ZM (2002) Catheter therapy of Swiss cheese ventricular septal defects using the Amplatzer muscular VSD occluder. Catheter Cardiovasc Interv 55(3):355–361
Hijazi ZM, Hakim F, Haweleh AA, Madani A, Tarawna W, Hiari A et al (2002) Catheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: initial clinical experience. Catheter Cardiovasc Interv 56(4):508–515
Butera G, Chessa M, Carminati M (2007) Percutaneous closure of ventricular septal defects. State of the art. J Cardiovasc Med (Hagerstown) 8(1):39–45
Yang R, Sheng Y, Cao K, Kong X, Xu D, Yong Y et al (2011) Transcatheter closure of perimembranous ventricular septal defect in children: safety and efficiency with symmetric and asymmetric occluders. Catheter Cardiovasc Interv 77(1):84–90
Cao H, Chen Q, Zhang GC, Chen LW, Li QZ, Qiu ZH (2011) Intraoperative device closure of perimembranous ventricular septal defects in the young children under transthoracic echocardiographic guidance; initial experience. J Cardiothorac Surg 6:166
Thanopoulos VTGEN et al (2020) Abstracts: PICS-AICS Virtual Symposium September 10–12, 2020. Pediatr Cardiol 41(6):1248–1298
Yang P, Wu Z, Liu Z, Zhang J, Zhou H, Ji X et al (2021) Unplanned surgery after transcatheter closure of ventricular septal defect in children: causes and risk factors. Front Pediatr. https://doi.org/10.3389/fped.2021.772138
Fu YC, Bass J, Amin Z, Radtke W, Cheatham JP, Hellenbrand WE et al (2006) Transcatheter closure of perimembranous ventricular septal defects using the new amplatzer membranous VSD occluder. J Am Coll Cardiol 47(2):319–325
Ghosh S, Mukherji A, Chattopadhyay A (2020) Percutaneous closure of moderate to large perimembranous ventricular septal defect in small children using left ventricular mid-cavity approach. Indian Heart J 72(6):570–575
Yang J, Yang L, Yu S, Liu J, Zuo J, Chen W et al (2014) Transcatheter versus surgical closure of perimembranous ventricular septal defects in children: a randomized controlled trial. J Am Coll Cardiol 63(12):1159–1168
Wang L, Cao S, Li J, Yang L, Liu Y, Ren J et al (2012) Transcatheter closure of congenital perimembranous ventricular septal defect in children using symmetric occluders: an 8-year multiinstitutional experience. Ann Thorac Surg 94(2):592–598
Carminati M, Butera G, Chessa M, Drago M, Negura D, Piazza L (2005) Transcatheter closure of congenital ventricular septal defect with amplatzer septal occluders. Am J Cardiol 96(12):52–58
el Said HG, Bratincsak A, Gordon BM, Moore JW (2012) Closure of perimembranous ventricular septal defects with aneurysmal tissue using the Amplazter Duct Occluder I: lessons learned and medium term follow up. Catheter Cardiovasc Interv 80(6):895–903
Guo W, Li Y, Yu J, Li J, Sun L, Shi J et al (2020) Transcatheter closure of perimembranous ventricular septal defect with aneurysm: radiologic characteristic and interventional strategy. J Interv Cardiol 2020:1–10
Saurav A, Kaushik M, Mahesh Alla V, White MD, Satpathy R, Lanspa T et al (2015) Comparison of percutaneous device closure versus surgical closure of peri-membranous ventricular septal defects: a systematic review and meta-analysis. Catheter Cardiovasc Interv 86(6):1048–1056
Jiang D, Han B, Zhao L, Yi Y, Zhang J, Fan Y et al (2021) Transcatheter device closure of perimembranous and intracristal ventricular septal defects in children: medium- and long-term results. J Am Heart Assoc. https://doi.org/10.1161/JAHA.120.020417
Wu H, Qin Y, Zhao X, Hu J, Zheng X, Wang E et al (2009) Transcatheter closure of multi-hole perimembranous VSD with aneurysm: 3-year follow-up study. Clin Res Cardiol 98(9):563–569
Narin N, Pamukcu O, Tuncay A, Baykan A, Sunkak S, Tasci O et al (2018) Percutaneous ventricular septal defect closure in patients under 1 year of age. Pediatr Cardiol 39(5):1009–1015
Funding
Open Access funding provided by the IReL Consortium.
Author information
Authors and Affiliations
Contributions
DA, DPK and NL wrote the main manuscript text and prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alshahrani, D., Linnane, N., McCrossan, B. et al. Transfemoral Perimembranous Ventricular Septal Defect Device Closure in Infants Weighing ≤ 10 kg. Pediatr Cardiol 44, 1176–1182 (2023). https://doi.org/10.1007/s00246-023-03100-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-023-03100-5