Abstract
The aim of the present study was to assess the ability of the biomarkers neuron-specific enolase (NSE) and S100 calcium-binding protein b (S100b) to predict 30 day mortality in children resuscitated from cardiac arrest (CA). It was a prospective observational study at a single tertiary heart centre. Consecutive children were admitted after resuscitated in-hospital and out-of-hospital CA. Levels of NSE and S100b were analyzed from 12 to 24 hours, from 24 to 48 hours, and from 48 to 72 hours after admission. The primary endpoint was 30-day mortality. Differences in biomarker levels between survivors and non-survivors were analyzed with the Mann-Whitney U test. Receiver operating characteristics (ROC) curves were applied to assess the predictive ability of the biomarkers and the areas under the ROC curves (AUC) were presented. A total of 32 resuscitated CA patients were included, and 12 (38%) patients died within 30 days after resuscitation. We observed significantly higher levels of NSE and S100b in non-survivors compared to survivors at all timepoints from 12 to 72 hours after CA. NSE achieved AUCs from 0.91–0.98 for prediction of 30 day mortality, whereas S100b achieved AUCs from 0.93–0.94. An NSE cut-off of 61 μg/L sampled between 12–24 hours from admission achieved a sensitivity of 80% and a specificity of 100% for prediction of 30 day mortality. In children resuscitated from CA, the biomarkers NSE and S100b appear to be solid predictors of mortality after 30 days.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Survival rates in children with cardiac arrest (CA) are poor, even if return of spontaneous circulation (ROSC) is achieved [1, 2]. Survival rates are reported as low as 30% after in-hospital CA (IHCA) and 8% after out-of-hospital CA (OHCA) [1, 3]. The primary cause of severe morbidity and mortality after resuscitated CA is neurological injury occurring as part of the post-CA syndrome [1, 4].
In patients remaining unconscious after ROSC, the decision whether to continue treatment or not, and for how long, is notoriously difficult [5, 6], as prognosis depends on a multitude of factors, including the cause of CA, time to ROSC, individual patient characteristics such as age and comorbidities. No present test or imaging are sufficiently accurate to be recommended as a stand-alone prognostication tool by contemporary guidelines that advocate for a multimodal prognostication strategy [7]. However, precision of individual prognostication tools after resuscitated CA has been sparsely investigated in children. Furthermore, a number of currently applied prognostic tests such as neurologic examination and electroencephalography (EEG) may be affected by sedatives which are frequently used in the post-resuscitation phase [8, 9]. As such, a biomarker with a high precision for prediction of poor outcome after resuscitated CA in children could be of great benefit.
Neuron-specific enolase (NSE) and S100 calcium-binding protein b (S100b) are relatively novel markers of cerebral injury [10, 11]. In adults, several studies have shown NSE to be a predictor of death and poor neurological outcome after CA [6, 12, 13]. A similar association has been reported for S100b, albeit less strong compared to NSE [5, 14]. Whether NSE and/or S100b are useful for prediction of outcome after CA in children has been sparsely investigated [1, 3, 15]. Accordingly, the aim of the present paper was to investigate the predictive ability of NSE and S100b in children resuscitated from CA for mortality and neurological outcome.
Materials and Methods
Study Design and Setting
The present study is a prospective analysis from a tertiary heart center. Consecutive patients younger than 18 years were included in the time period from February 2011 to December 2021. Patients being resuscitated from IHCA as well as patients being admitted after resuscitated OHCA were included. CA was defined as external chest compressions for one minute or more [1, 3]. Only patients remaining unconscious after ROSC were included. Patient characteristics were collected from electronic patient charts. All endpoints and analyses were defined a priori. The study was approved by the center’s institutional review board and determined to meet the requirements of health surveillance and quality control. Biomarkers were measured as part of clinical routine, and due to the observational nature of the study, a waiver of informed consent was granted.
Patients
After resuscitation, all patients were admitted to an intensive care unit (ICU) for post-resuscitation care. On admission to the ICU, an arterial blood gas was drawn and analyzed. As part of clinical routine, blood was analyzed for NSE and S100b daily from 12 to 72 h after admission. Serial NSE and S100b analyses were being adopted gradually as routine procedure at our institution during the inclusion period. Accordingly, blood for analysis of NSE and S100b was frequently drawn at fixed rounds, simultaneous with blood for analyses of other biochemistry. Thus, NSE and S100b levels were not measured at specific timepoints after ROSC, but rather in the following time periods: 12 to 24 h, 24 to 48 h, and 48 to 72 h after ROSC (or ECMO initiation), Post-resuscitation care was provided according to institutional standard procedures following contemporary guidelines. Further diagnostic examinations, including EEG, somatosensory evoked potentials (SSEP) or cerebral imaging, were not routinely used but were initiated at attending physicians’ discretion.
Biomarker Analysis
Analyses of NSE were performed with the Cobas 8000, e602 module, using an electrochemiluminescence immunoassay (ECLIA) kit (Roche Diagnostics), with a range from 0.1 to 370 μg/L and a between-run precision at 11 μg/L and 85 μg/L of 8% and 7%, respectively. Analyses of S100b were performed with the Cobas 8000, e602 module, using an ECLIA kit (Roche Diagnostics), with a range from 0.02 to 39 μg/L and a between-run precision at 0.09 μg/L and 3.3 μg/L of 6%.
The department of Biochemistry applied specific equations to correct measured levels of NSE for hemolysis.
Outcome Measurements
The primary outcome was 30-day mortality. The secondary outcome was neurologic outcome after 180 days, assessed by the pediatric cerebral performance category (PCPC) score [16]. Neurological outcome was dichotomized into good neurological outcome (a PCPC score of one to three) or poor neurological outcome (a PCPC score of four to six) equivalent to severe disability, coma or death [3, 15]. Outcomes were adjudicated by review of medical files by two of the authors of this manuscript.
Statistical Analyses
Assuming a mean (SD) NSE level of 90 (60) µg/L after 48 h in non-survivors and a mean (SD) NSE level of 25 (60) µg/L after 48 h in survivors, the study would achieve a power of 0.84 at a significance level of 0.05 if 30 patients were included (sample size calculation completed with the non-parametric Mann–Whitney U-test). The assumed NSE levels were based on an adult population with out-of-hospital CA [17]. Categorical variables were presented as numbers (n) and percentages (%) and differences between strata were compared with the Chi-Square test or Fisher’s Exact test as appropriate. Continuous variables were presented as mean ± standard deviation (SD) if normally distributed or as median (inter-quartile range, IQR) if skewed and differences between strata were compared with the Student’s t-test or the Mann–Whitney U test as appropriate. Baseline characteristics were presented stratified by 30-day survival. Median levels of NSE and S100b were presented at each time point after stratification by 30-day survival. To assess the predictive ability of NSE and S100b, receiver operating characteristics (ROC) were applied, and the area under the curves (AUC) were presented. The Hosmer and Lemeshow test were applied to assess the goodness-of-fit for the models. In addition, for a set specificity of 100% (i.e., a false-positive rate of 0), we presented the cut-off values with corresponding predictive values for each biomarker measurement. All tests were two-sided with a significance level of 0.05. Statistical analyses are done with SAS Enterprise Guide software, version 7.1.
Results
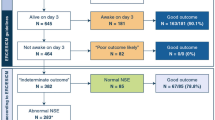
A total of 32 patients were included in the study. A total of 10 (31%) patients were female, median age was 12 (4–16) years with a range from 0 to 17 years. Within the first 30 days from admission 12 (38%) patients died. Non-survivors had a significantly higher level of blood glucose after admission compared to survivors (Table 1). Non-survivors had significantly higher lactate levels 12 h after admission compared to survivors. We found no other significant differences in baseline characteristics between non-survivors and survivors (Table 1).
No patients died between 30 and 180 days from admission. After 180 days, 14 (44%) patients had a PCPC score of four or higher, i.e., a poor neurologic outcome including death.
Neuron-Specific Enolase Levels
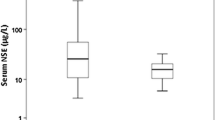
A total of 29 (91%) had NSE measured between 12 and 24 h, 29 (91%) had NSE measured between 24 and 48 h, and 21 (66%) had NSE measured between 48 and 72 h. While decreasing in survivors, median NSE levels increased from 12 to 72 h from admission in non-survivors (p < 0.001). Median NSE levels were significantly higher in non-survivors versus survivors at all time points (Fig. 1).
The AUC for prediction of 30-day mortality was 0.91 (0.75–1.0) for NSE measured after 12 to 24 h, 0.97 (0.92–1.0) for NSE measured after 24 to 48 h, and 0.98 (0.93–1.0) for NSE measured after 48 to 72 h. The Hosmer and Lemeshow goodness-of-fit tests were insignificant for all three models (p = 0.27–0.99).
For a set specificity of 100% (i.e., a false-positive rate of 0), the cut-off levels of NSE were 61 μg/L when measured between 12 and 24 h, 98 μg/L when measured between 24 and 48 h, and 59 μg/L when measured between 48 and 72 h (Table 2).
The AUC for prediction of poor neurologic outcome after 180 days was 0.90 (0.76–1.0) for NSE measured after 12 to 24 h, 0.92 (0.80–1.0) for NSE measured after 24 to 48 h, and 0.88 (0.72–1.0) for NSE measured after 48 to 72 h. The cut-offs with corresponding predictive values are presented in Table 3.
S100b Levels
A total of 31 (97%) had S100b measured between 12 and 24 h, 31 (97%) had S100b measured between 24 and 48 h, and 22 (69%) had S100b measured between 48 and 72 h. Median levels of S100b at all time points were significantly higher in non-survivors versus survivors (Fig. 2). The area under the ROC curves for prediction of 30-day mortality was 0.93 (0.84–1.0) for S100b measured after 12 to 24 h, 0.94 (0.86–1.0) for S100b measured after 24 to 48 h, and 0.94 (0.85–1.0) for S100b measured after 48 to 72 h. The Hosmer and Lemeshow goodness-of-fit tests were insignificant for all three models (p = 0.25–0.84).
For a set specificity of 100% (i.e., a false-positive rate of 0), the cut-off levels of S100b were 2.0 μg/L when measured between 12 and 24 h, 3.3 μg/L when measured between 24 and 48 h, and 0.98 μg/L when measured between 48 and 72 h (Table 2).
The AUC for prediction of poor neurologic outcome after 180 days was 0.89 (0.77–1.0) for S100b measured after 12 to 24 h, 0.89 (0.77–1.0) for S100b measured after 24 to 48 h, and 0.84 (0.65–1.0) for S100b measured after 48 to 72 h. The cut-offs with corresponding predictive values are presented in Table 3.
Blood Glucose and Lactate Levels
The AUC for prediction of 30-day mortality was 0.82 (0.63–1.0) for blood glucose measured at admission. The AUC for prediction of 30-day mortality was 0.69 (0.50–0.87) for lactate measured at admission and 0.74 (0.54–0.93) for lactate measured 12 h after admission.
Discussion
The primary finding of this study was that NSE and S100b levels measured 12 to 72 h after admission in children resuscitated from CA were strong predictors of 30-day mortality and of poor neurologic outcome after 180 days.
NSE is mainly found in neuronal and neuroendocrine cells and S100b in astrocytes and Schwann cells [18, 19]. Following cerebral injury these cells are damaged and the biomarkers are released to the blood. Their level in serum is related to the extent of cerebral injury and therefore potentially able to predict outcome [1, 15]. NSE is suggested to be used for as part of a multimodal prognostication strategy after CA, where the majority of mortality can be attributed to anoxic–ischemic neurological injury [7]. S100b is mainly applied after traumatic brain injury related to diffuse axonal injury [10]. However, extra-central nervous system sources can confound biomarker analysis [3]. Increased serum levels of NSE can be caused by hemolysis as well as presence of small cell lung carcinoma and neuroendocrine tumors [6]. Increased serum levels of S100b is reported after cardiac surgery as well as after hypoperfusion in patients with sepsis [3].
Three previous studies have investigated the predictive ability of NSE and S100b in children with CA. The 30-day survival rate was 62% in the present study, which is comparable to the survival rates reported in the three other studies. The largest and most recent study by Kramer et al. included 95 children with CA from two departments of congenital heart disease and congenital heart surgery [1]. The primary outcome was PCPC score at discharge, and the study reported AUC’s from 0.73 to 0.83 for NSE and from 0.78 to 0.82 for S100b measured at 24 to 72 h. The lower predictive ability of the biomarkers compared to the present study may be explained by one or more of the following factors. In contrast to the present study (which included one patient with congenital heart disease), Kramer et al. primarily included post-surgical patients with congenital heart disease. In this population, death caused by circulatory failure is likely to be more prevalent compared to an all-comer population of mixed causes of CA, where death caused by neurologic injury may be more prevalent. Further, in the study by Kramer et al., a large proportion of patients underwent cardio-pulmonary bypass, and elevated NSE levels caused by hemolysis may have confounded the results. Finally, in a larger sample size, the higher likelihood of outliers will generally lead to lower predictive values for a given biomarker. A prospective study by Topjian et al. included 35 patients resuscitated after CA of mixed origin [3]. The study reported an area under the ROC curve of 0.85 for NSE measured after 48 h versus poor neurological outcome defined as the PCPC score before discharge. In 43 children with resuscitated CA, Fink et al. reported an AUC of 0.79 for NSE versus mortality and an AUC of 0.91 for S100b versus mortality [15]. In contrast to the present study, biomarker values from multiple timepoints were pooled for assessment of ROC.
The paper by Fink et al. reported an NSE cut-off value of 53 μg/L at a false-positive rate of 0 when measured after 24 h and a cut-off value of 77 μg/L when measured after 48 h in children after CA [15]. In contrast, Kramer et al. reported higher cut-off values of NSE at 133 μg/L when measured after 24 h, 118 μg/L when measured after 48 h, and 112 μg/L when measured after 72 h [1]. The higher cut-off values, as well as the decreasing trend of the cut-off values, may be explained by hemolysis occurring during index heart surgery, i.e., prior to CA.
Contemporary guidelines for post-resuscitation care in adults suggest the use of NSE as part of a multimodal prognostication strategy. Here, an NSE cut-off above 60 μg/L after 48 and/or 72 h is suggested as a marker of poor prognosis [7]. Accordingly, the suggested cut-off values by the present article correspond well with the suggested cut-off value for adults. Importantly, adult guidelines emphasize that a multimodal prognostication approach should be applied to limit the risk of a false-positive, i.e., withdrawal in a patient with a potential good outcome. The need to minimize the risk of false-positive prognostication is no less important in pediatric CA, and it is unlikely that any one test will be sufficiently accurate for prognostication. However, NSE may prove an important part in future multimodal prognostication strategies. Current guidelines do not recommend the use of S100b for prognostication after adult CA, as the predictive ability is inferior compared to NSE [7]. This corresponds to the results of the present paper. Guidelines for prognostication after resuscitated CA in children simply suggest that biomarkers may be indicative, but that cut-off values remain unknown [20].
Compared to other modes of prognostication after CA biomarkers have the advantage of being independent of sedatives which often confound outcome prediction in resuscitated patients treated in the intensive care unit [7, 21]. Further, biomarkers have the advantage that the clinical team can be blinded for the results, and as such, biomarkers may serve as potential outcome markers in future clinical trials.
The presented results should be interpreted after consideration of the following limitations. The study was observational in design, and a sample size of 32 children limits the probability of identifying outliers. The presented predictive abilities of the biomarkers are likely to be lower in a larger population with a higher likelihood of outliers, and the cut-off value will need to be evaluated in larger prospective studies prior to clinical application. There are many causes of CA and it is likely that the predictive ability of the biomarkers may depend on various other factors connected to the specific cause of CA. The present sample size is too small for conducting analyses on subgroups. Accordingly, the results should be considered hypothesis-generating.
Conclusion
In children resuscitated from CA, NSE, and S100b analyzed 12 to 72 h after admission are highly associated with 30-day mortality, and the biomarkers appear to be strong predictors of mortality. Future large-scale prospective studies are needed to further define cut-off points and to potentially combine biomarkers with clinical risk scores.
References
Kramer P, Miera O, Berger F, Schmitt K (2018) Prognostic value of serum biomarkers of cerebral injury in classifying neurological outcome after paediatric resuscitation. Resuscitation 122:113–120
López-Herce J, Del Castillo J, Matamoros M, Cañadas S, Rodriguez-Calvo A, Cecchetti C et al (2013) Factors associated with mortality in pediatric in-hospital cardiac arrest: a prospective multicenter multinational observational study. Intensive Care Med 39(2):309–318
Topjian AA, Lin R, Morris MC, Ichord R, Drott H, Bayer CR et al (2009) Neuron-specific enolase and S-100B are associated with neurologic outcome after pediatric cardiac arrest*. Pediatr Crit Care Med 10(4):479–90
Binks A, Nolan JP (2010) Post-cardiac arrest syndrome. Minerva Anestesiol 76(5):362–368
Wiberg S, Kjaergaard J, Kjærgaard B, Møller B, Nørnberg B, Marie A et al (2017) The biomarkers neuron-specific enolase and S100b measured the day following admission for severe accidental hypothermia have high predictive values for poor outcome. Resuscitation 2020(121):49–53
Wiberg S, Hassager C, Stammet P, Winther-jensen M, Thomsen JH, Erlinge D et al (2017) Single versus serial measurements of neuron-specific enolase and prediction of poor neurological outcome in persistently unconscious patients after out-of-hospital cardiac arrest—a TTM-trial substudy. PLoS ONE 12:e0168894
Nolan JP, Sandroni C, Böttiger BW, Cariou A, Cronberg T, Friberg H et al (2021) European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: post-resuscitation care. Resuscitation 161:220–269
Mandel R, Martinot A, Delepoulle F, Lamblin M-D, Laureau E, Vallee L et al (2002) Prediction of outcome after hypoxic-ischemic encephalopathy: a prospective clinical and electrophysiologic study. J Pediatr 141(1):45–50
Drohan CM, Cardi AI, Rittenberger JC, Popescu A, Callaway CW, Baldwin ME et al (2018) Effect of sedation on quantitative electroencephalography after cardiac arrest. Resuscitation 124:132–137
Chabok SY, Moghadam AD, Saneei Z, Amlashi FG, Leili EK, Amiri ZM (2012) Neuron-specific enolase and S100BB as outcome predictors in severe diffuse axonal injury. J Trauma Acute Care Surg 72(6):1654–1657
Rosén H, Sunnerhagen KS, Herlitz J, Blomstrand C, Rosengren L (2001) Serum levels of the brain-derived proteins S-100 and NSE predict long-term outcome after cardiac arrest. Resuscitation 49(2):183–191
Stammet P, Collignon O, Hassager C, Wise MP, Hovdenes J, Åneman A et al (2015) Neuron-specific enolase as a predictor of death or poor neurological outcome after out-of-hospital cardiac arrest and targeted temperature management at 33°C and 36°C. J Am Coll Cardiol 65(19):2104–2114
Sandroni C, Cavallaro F, Callaway CW, D’Arrigo S, Sanna T, Kuiper MA et al (2013) Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 2: patients treated with therapeutic hypothermia. Resuscitation 84(10):1324–38
Tiainen M, Roine RO, Takkunen O (2003) Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke 34:2881–2886
Fink EL, Berger RP, Clark RSB, Watson RS, Angus DC, Richichi R et al (2014) Serum biomarkers of brain injury to classify outcome after pediatric cardiac arrest*. Crit Care Med 42(3):664–674
Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M (2000) Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med 28(7):2616–2620
Wiberg S, Hassager C, Schmidt H, Thomsen JH, Frydland M, Lindholm MG et al (2016) Neuroprotective effects of the glucagon-like peptide-1 analog exenatide after out-of-hospital cardiac arrest: a randomized controlled trial. Circulation 134(25):2115–2124
Reiber H (2003) Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci 21(3–4):79–96
Hu J, Ferreira A, Van Eldik LJ (1997) S100beta induces neuronal cell death through nitric oxide release from astrocytes. J Neurochem 69(6):2294–2301
Van de Voorde P, Turner NM, Djakow J, de Lucas N, Martinez-Mejias A, Biarent D et al (2021) European Resuscitation Council guidelines 2021: paediatric life support. Resuscitation 161:327–387
Samaniego EA, Mlynash M, Caulfield AF, Eyngorn I, Wijman CAC (2011) Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit Care 15(1):113–119
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bangshøj, J., Liebetrau, B., Wiberg, S. et al. The Value of the Biomarkers Neuron-Specific Enolase and S100 Calcium-Binding Protein for Prediction of Mortality in Children Resuscitated After Cardiac Arrest. Pediatr Cardiol 43, 1659–1665 (2022). https://doi.org/10.1007/s00246-022-02899-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-022-02899-9