Abstract
After the arterial switch operation (ASO) for transposition of the great arteries (TGA), many patients have an impaired exercise tolerance. Exercise tolerance is determined with cardiopulmonary exercise testing by peak oxygen uptake (VO2peak). Unlike VO2peak, the oxygen uptake efficiency slope (OUES) does not require a maximal effort for interpretation. The value of OUES has not been assessed in a large group of patients after ASO. The purpose of this study was to determine OUES and VO2peak, evaluate its interrelationship and assess whether exercise tolerance is related to ventricular function after ASO. A cardiopulmonary exercise testing, assessment of physical activity score and transthoracic echocardiography (fractional shortening and left/right ventricular global longitudinal peak strain) were performed to 48 patients after ASO. Median age at follow-up after ASO was 16.0 (IQR 13.0–18.0) years. Shortening fraction was normal (36 ± 6%). Left and right global longitudinal peak strain were reduced: 15.1 ± 2.4% and 19.5 ± 4.5%. This group of patients showed lower values for all cardiopulmonary exercise testing parameters compared to the reference values: mean VO2peak% 75% (95% CI 72–77) and mean OUES% 82(95% CI 77–87); without significant differences between subtypes of TGA. A strong-to-excellent correlation between the VO2peak and OUES was found (absolute values: R = 0.90, p < 0.001; normalized values: R = 0.79, p < 0.001). No correlation was found between cardiopulmonary exercise testing results and left ventricle function parameters. In conclusion, OUES and VO2peak were lower in patients after ASO compared to reference values but are strongly correlated, making OUES a valuable tool to use in this patient group when maximal effort is not achievable.
Similar content being viewed by others
Introduction
After correction with the arterial switch operation (ASO), long-term survival and outcome of patients with transposition of the great arteries (TGA) are usually good. However, residual lesions, including right ventricular outflow tract obstruction, aortic root dilatation, aortic insufficiency and left ventricular dysfunction with or without coronary artery abnormalities can contribute to increased morbidity [1, 2]. Moreover, impaired exercise tolerance has been described in patients after ASO, sometimes already being present at a young age [3,4,5,6,7,8]. Contributing factors to reduced exercise tolerance have been shown to include chronotropic incompetence, narrowing of the main pulmonary artery with or without pulmonary branch obstruction, coronary abnormalities, ventricular dysfunction and longer follow-up time after ASO [5,6,7,8,9,10]. To test exercise performance, the gold standard is the cardiopulmonary exercise test with measurement of the maximal oxygen consumption (VO2peak) [11], but it requires the capacity to perform maximal exercise for its interpretation. In certain patient groups, e. g. young children, the required maximal exercise during a cardiopulmonary exercise test will often not be reached due to motivational aspects. In addition, patients with mental disability or with certain cardiovascular diseases may have reduced capacity to fulfil the required maximum exercise. These considerations make the use of submaximal exercise parameters such as the ventilatory efficiency (VE/VCO2slope) and the oxygen uptake efficiency slope (OUES) potentially valuable [12]. OUES has been investigated in both healthy subjects and patients with congenital heart defects over a wide age range and it was shown to be an objective and effort-independent cardiopulmonary exercise test parameter, strongly correlated to VO2peak [13,14,15,16]. However, its value in patients after ASO has not been previously determined. Therefore, the aim of the present study was to correlate OUES as a submaximal exercise parameter to VO2peak as a maximal exercise parameter in a group of patients after ASO. In addition, we studied whether exercise tolerance could be related to ventricular function or right ventricle outflow tract obstruction as assessed by echocardiography.
Material and Methods
Forty-eight patients with TGA with intact ventricular septum or with ventricular septal defect after ASO were included. Patients with complex TGA including Taussig-Bing anomaly, prior left ventricular outflow tract obstruction or aortic arch obstruction were excluded. A cardiopulmonary exercise test, assessment of physical activity score and a transthoracic echocardiogram were performed.
All patients performed a progressive cardiopulmonary exercise test on an electronically braked cycle ergometer (GE Healthcare eBike Comfort, Freiburg, Germany) up to exhaustion. A facemask (Hans Rudolph, Kansas City, MO, USA) connected to a flowmeter (Triple V volume transducer) and a computerized gas analyser (Jaeger MasterScreen CPX, CareFusion GmbH, Hoechberg, Germany) which calculated breath-by-breath minute ventilation (VE), oxygen uptake (VO2), carbon dioxide production (VCO2) and respiratory exchange ratio (RER, defined as the ratio VCO2/VO2) in 10 s intervals were used. Heart rate (HR) was continuously monitored through a twelve-lead electrocardiogram and blood pressure was determined every 2 min by sphygmomanometry. A 3 min warm-up phase (unloaded cycling) was followed by a continuous incremental bicycle protocol with a work rate increment of 10, 15 or 20 W/min depending on the height (< 125 cm, 125–150 cm or > 150 cm) according to Godfrey protocol [17]. The patients had to maintain a pedalling rate between 60 and 80 revolutions/min and were encouraged to perform to exhaustion. The cardiopulmonary exercise test could be terminated by the patient in case of discomfort or by the physician in case of ECG changes, excessive breathing pattern or otherwise. Test with a peak RER (RERpeak) of ≥ 1.00 were included for analysis.
The RERpeak was calculated as the average of 2 highest consecutive achieved RER values in 10 s during peak work rate. Peak work rate was defined as the maximum work rate achieved and finished (1 min completed) and the %predicted value was calculated [18, 19]. HR at rest was measured after at least 3 min in a seated position and HR peak was calculated as the highest value achieved during at least 10 s in peak work rate. Then the %predicted value was calculated with the formula [200-age], being abnormal < 85% [11]. HR reserve was defined as HR peak minus HR rest. HR was also recorded at 1 and 2 min after cessation of the cardiopulmonary exercise test (HR01′ and HR02′). HR recovery was calculated as the difference between HR peak and HR01′ and HR peak and HR02′. The relative decrement in HR (HR01% and HR02%) was calculated as (HR recovery/HR reserve) × 100%.
VO2peak (ml/min) was calculated as the average of 2 highest consecutive achieved VO2 values in 10 s during WRpeak. Reference values were used for the interpretation of the results from the exercise tests and for calculation the %predicted value of VO2peak (VO2peak%) [20]. A VO2peak% value was considered abnormal < 85%. VE/VCO2slope is the slope of the linear regression of VE and VCO2 during the entire period of the test. The O2pulse is the VO2 divided by HR and the maximal O2pulse (O2pulsemax) was calculated as the average of the highest two consecutive O2pulse values during WRpeak. The data of Ten Harkel et al. [18] was used to calculate the % predicted values of O2 pulse. OUES was calculated by the linear least squares regression of the VO2 on the common logarithm of the VE by the equation VO2 = alog (VE) + b, where the constant ‘a’ is the regression coefficient OUES [12]. Absolute values, %predicted values and values per body weight were represented. Weight and height were obtained and body surface area (BSA) and body mass index (BMI) were calculated by using the Dubois equation. The %predicted value of OUES (OUES%) was determined using the previous described formulas based on reference normal values adjusted for age and sex [13, 16].
A lifestyle interview was performed to evaluate patients’ weekly exercise behaviour according to the previously described method [21]. In short, patients were queried on their voluntary exercise behaviour (e.g. swimming, fitness, tennis, jogging, soccer) and physical activities related to transportation (cycling, walking) and compulsory physical education classes. Only activities done for at least 6 months and more than 3 months per year were included. Each exercise activity was converted into a metabolic equivalent task (MET) score [22], and a weekly MET score (METhours/week) was calculated (i.e. MET scores multiplied by the duration of activities and summed).
Transthoracic echocardiography was performed using a commercially available system (Vivid-7.0.0, General Electric Vingmed Ultrasound, Horten, Norway) and images were stored in digital format. Off-line analyses were made using EchoPac version 11.1.8 (General Electric Vingmed). Left ventricular (LV) systolic performance was assessed using LV fractional shortening (FS) in M-mode recordings of the parasternal LV long axis view. LV internal diameter at end-diastole (LVIDd) and LV internal diameter at end-systole (LVIDs) were assessed and FS was calculated as follows: ((LVIDd-LVIDs)/LVIDd) × 100%. Left and right ventricular global longitudinal strain (GLS) was obtained from the apical 4-chamber view using speckle-tracking strain analysis as previously described and according to the international guidelines [23, 24]. In patients with a repaired VSD, care was taken to exclude the patch area from the strain analysis.
Tricuspid regurgitation was identified using colour-flow Doppler in the apical 4-chamber view. Estimation of the right ventricular pressure was performed using CW Doppler by placing the ultrasound beam aligned to the tricuspid regurgitation when it was present. To assess the severity of right ventricular outflow tract obstruction, the maximal right ventricular outflow tract velocity with CW Doppler across main pulmonary artery and right and left pulmonary arteries was measured. Stenosis was graded based on the greatest maximal velocity (Vmax): mild, Vmax = 2–3 m/s; moderate, Vmax = 3–4 m/s; or severe, Vmax > 4 m/s.
Data analysis was performed using SPSS Statistics software (v.25.0 IBM SPSS, Chicago, IL). Variables were tested for normal distribution using the Shapiro–Wilk test. Continuous data were expressed as mean ± standard deviation (SD) or as median and inter-quartile range (IQR) where suitable. The paired samples t-test or the Mann–Whitney U test, in case of non-normality, were used to assess differences in cardiopulmonary exercise test or echocardiographic parameters between sex and diagnosis (with intact ventricular septum or with ventricular septum defect). The exercise test results were expressed relatively to the reference values, as % of predicted value (100% would mean equal to reference value) and represented as mean with 95% of confidence interval (CI). To test whether the values of patients differed from their reference values, the one sample t-test was used. Correlations between the exercise test parameters and the echocardiographic parameters were calculated as Pearson or Spearman correlation coefficient depending on data distribution. Correlations between age and VO2peak and OUES were performed by Pearson correlation as well. p values < 0.05 were accepted as statistically significant.
Results
Table 1 shows the general characteristics of the study group. Forty-eight patients were included, 37 of them (77.1%) had intact ventricular septum and 11 had ventricular septum defect. One-stage ASO was performed in 95.7% of the patients; in two patients a two-stage approach was performed. Median age at ASO was 6 days (IQR 4–9) and the median age at follow-up was 16.0 (IQR 13.0–18.0) years post-ASO.
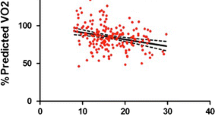
All patients exercised to exhaustion with an RER > 1.0 without any adverse events. Cardiopulmonary exercise test results are depicted in Table 2. TGA patients showed on average lower values for all exercise test parameters compared to reference values from a healthy dataset [13, 16, 20], as reflected by %predicted values: VO2peak% = mean 75% (95% CI 72–77), p < 0.001; and OUES% = mean 82% (95% CI 77–87), p < 0.001. O2pulsemax was also decreased with a percentage predicted of 79% (p < 0.001). No significant differences in cardiopulmonary test parameters were found between patients with intact ventricular septum or with ventricular septum defect. As expected, female patients had significant lower VO2peak and OUES compared to male TGA patients. The %predicted values for male and female of VO2peak (VO2peak%: male = mean 74% (95% CI 71–77) vs. female = mean 77% (95% CI 70–84); p = 0.358 and OUES (OUES%: male = 84% (95% CI 78–89) vs. female = mean 77% (95% CI 68–87); p = 0.505 were not significantly different. There was a significant negative linear relationship between age and OUES% (R = -0.39, p = 0.006) (Fig. 1) but not between age and VO2peak%. The relation between the oxygen uptake and the minute ventilation in two TGA patients with good and bad exercise performance and the Wasserman nine panel plots of this patients are depicted in Figs. 2 and 3 as an illustration. Figure 4 shows an excellent and strong correlation respectively between the VO2peak and OUES, for both the absolute and the normalized data (R = 0.90, p < 0.001 and R = 0.79 p < 0.001, respectively).
OUES depicted in two patients after arterial switch operation during a cardiopulmonary exercise testing. Panel a 12-year-old male patient with OUES = 1499 ml/min (68% of the predicted value). Panel b 17-year-old male patient with OUES = 2934 ml/min (90% of the predicted value). A steeper slope represents a more efficient oxygen uptake: the higher the OUES value, the higher the slope is, meaning that a smaller minute ventilation is needed for a determined oxygen uptake. LogVE common logarithm of minute ventilation, VE minute ventilation, VO2 oxygen uptake
Wasserman 9-plot of the same two patients of Fig. 2. Panel a RERpeak 1.2, WRp 135 W (92% of the predicted value), HRpeak 187 bpm (99% of the predicted value), VO2peak 1616 ml/min (73% of the predicted value), VE 28.03, O2pulsemax 8.65 ml/bpm (68% of the predicted value). Panel b RERpeak 1.2, WRp 300 W (122% of the predicted value), HRpeak 196 bpm (107% of the predicted value), VO2peak 3564 ml/min (96% of the predicted value), VE 29.7, O2pulsemax 18.3 ml/bpm (90% of the predicted value). HRpeak maximal heart rate at peak exercise, O2pulsemax maximal O2 pulse, RERpeak respiratory exchange ratio at peak exercise, VO2peak oxygen uptake at peak exercise, VE ventilatory efficiency, WRpeak peak work rate
Echocardiographic results are presented as well in Table 1. All patients were in sinus rhythm. LV systolic function represented by the FS was on average 36% ± 6 and above 30% in 94% of all TGA patients. LV and RV GLS were − 15.1% ± 2.4 and − 19.5% ± 4.5 respectively. There was no significant difference in the GLS between patients with IVS and VSD (IVS: − 14.3 ± 2.4 vs VSD: − 13.6 ± 3.9, p 0.45). Stenosis in pulmonary arteries was present in 83.3% of patients (64.6% mild and 18.8% moderate stenosis). No correlation was found between exercise test results (VO2peak and OUES) and ventricular function parameters (FS, LV and RV GLS), the maximal CW Doppler measured in pulmonary arteries or the maximal gradient of the tricuspid regurgitation. Moreover, there were no significant differences between the 24 patients with the highest and the 24 patients with the lowest VO2peak/kg values in the Vmax across de RVOT (2.67 m/s vs 2.48 m/s, p = 0.312).
Average weekly MET score was 52.5 ± 26.2, without significant differences between males and females (male = 54.6 ± 27.8 vs. female = 47.3 ± 23.3, p = 0.581). One patient scored < 21 METhour/week. Weekly MET score showed a significant moderate correlation with the VO2peak% (R = 0.55, p = 0.009).
Discussion
The results of this study demonstrate that patients post-ASO have diminished exercise capacity, depicted in both lower VO2peak and OUES. OUES and VO2peak showed an excellent correlation which supports the value and importance of OUES as an effort independent cardiopulmonary exercise test parameter. No correlation was found between VO2peak or OUES and ventricular function parameters (FS, LV and RV GLS) or the maximal CW Doppler gradient measured in pulmonary arteries.
As previously mentioned in certain patients groups such as young children or older patients with multiple morbidities maximal exercise during cardiopulmonary exercise test will often not be reached. Therefore, it would be of importance if exercise parameters during submaximal exercise would give the same information as the gold standard parameter VO2peak. In previous studies it has been shown that OUES, a parameter obtained during submaximal exercise, is an objective effort-independent parameter to evaluate cardiopulmonary fitness [12, 14, 15, 25, 26]. To our knowledge OUES has not been previously evaluated in a group of children with congenital heart defects and in particular children with TGA after ASO. In the present study we found an excellent correlation between OUES and VO2peak and between indexed to weight OUES and VO2peak in TGA patients after ASO. These findings make it possible to perform submaximal exercise and gain similar information as VO2peak in conditions where maximal exercise is less appropriate as in young children or during e.g. a postoperative course after reoperations.
A reduced exercise performance was present in our paediatric population of TGA patients, which confirms the results of previous studies [3, 5,6,7,8]. Both the submaximal value of OUES as well as the VO2peak were reduced as compared to a reference population. Cardiopulmonary exercise testing with the use of continuous measurement of HR, VO2 and VCO2 assess the integrity of the pulmonary system, cardiovascular system, autonomic nervous system and peripheral muscles. In patients with congenital heart disease all these factors may play a role in the presence of reduced exercise capacity. The importance of an intact autonomic nervous system in congenital heart defects patients is revealed by the fact that a reduction in parasympathetic nervous activity may contribute to increased mortality. HR recovery after cessation of exercise is mainly driven by the parasympathetic system. In the present study HR recovery after 1 min was 31%, which corresponds to the value obtained in healthy female subjects in other studies, but was a bit lower in males (31% vs 35%) [18, 27]. Sympathetic denervation after ASO has been postulated as one of the possible causes of reduced chronotropic competence in TGA patients [28]. However, in the present study we did not find a reduced maximal HR, which correlates to several studies [3, 6], although some other studies did find a reduced maximal heart rate [7, 8]. The % predicted value of O2pulsemax was reduced in our patients and this could reflect a reduced stroke volume at peak exercise [29].
Ventilatory efficiency as assessed by the VE/VCO2 slope is reduced in many congenital heart defects patients. This measure is highly related to long-term outcome. In our study the VE/VCO2slope of the TGA patients was similar to the values of healthy children previously published (i.e. VE/VCO2slope of 30) [13, 18].
Systolic ventricular function is an important factor in exercise tolerance. Although we found a normal FS in this study, both LV and RV global longitudinal peak strain were reduced [24, 30]. Reduced LV longitudinal strain has also been found previously after ASO [31, 32]. A main residual lesion in TGA patients is often the presence of pulmonary stenosis, either of the main pulmonary artery or the pulmonary branches. In many adult patients after ASO, a decreased area in the main pulmonary artery and/or pulmonary branches has been reported to be correlated toVO2peak [9]. In addition, an abnormal (right/left) pulmonary blood flow distribution has also been associated with a decrease exercise capacity [9, 10], and other show that an increased right ventricle outflow tract velocity was correlated to a reduced VO2peak [5]. Although the presence of mild to moderate stenosis in pulmonary branches was common in this study, a correlation with lower VO2peak or OUES could not be detected.
It is as yet unclear if the exercise tolerance in TGA patients will decrease over time. In the present study we found a negative correlation between %OUES and age. However, another study did not find any correlation between age and exercise capacity [5]. Many studies have investigated the role of daily exercise behaviour and the effect of training in congenital heart defects patients. Also, TGA patients have found not to achieve the physical activity levels as recommended in guidelines [33], although other studies found similar levels of physical activity in TGA patients and healthy control subjects [8]. In the present study the weekly MET level was comparable to normal values. However, we observed lower weekly exercise behaviour significantly correlating to diminished exercise capacity. In a large review it was concluded that in children and young adults with congenital heart defects exercise is safe and an improvement of fitness after a physical exercise training programme can be obtained [34]. The findings of the correlation between weekly exercise behaviour and exercise capacity in this study suggests that this effect of training might be valuable in TGA patients as well, and performing regular physical activity in these patients should be stimulated.
Our study has several limitations. The correlation between age and exercise capacity may as well reflect the surgical era, notwithstanding operative techniques have not changed significantly during this period. The measurement of weekly exercise behaviour has been performed by questionnaires and therefore remain a subjective parameter. Measurement by accelerometer or continuous HR monitoring could have been more exact. And finally, although this paper presents one of the larger studies about TGA and exercise performance the power might be too low to find more subtle correlations as with e.g. systolic ventricular function.
In conclusion, we found a good correlation between OUES and VO2peak in a group of TGA patients after ASO, making OUES a valuable tool to use in this patient group when maximal effort is not possible. Still, longer-term follow-up studies are needed to determine whether OUES is a reliable parameter for determining exercise capacity in this group of patients and whether the correlation with the VO2peak remains consistent. The lower exercise performance in our group as assessed by both VO2peak and OUES might be multifactorial, including decreased parasympathetic activity, while other potential factors as pulmonary stenosis and systolic ventricular function may contribute, although not significantly correlated in the present study. Furthermore, deterioration in exercise capacity over age may play a role, while increasing daily exercise performance may contribute to stabilizing exercise capacity.
References
Fricke TA, d’Udekem Y, Richardson M, Thuys C, Dronavalli M, Ramsay JM, Wheaton G, Grigg LE, Brizard CP, Konstantinov IE (2012) Outcomes of the arterial switch operation for transposition of the great arteries: 25 years of experience. Ann Thorac Surg 94(1):139–145. https://doi.org/10.1016/j.athoracsur.2012.03.019
Khairy P, Clair M, Fernandes SM, Blume ED, Powell AJ, Newburger JW, Landzberg MJ, Mayer JE Jr (2013) Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation 127(3):331–339. https://doi.org/10.1161/CIRCULATIONAHA.112.135046
de Koning WB, van Osch-Gevers M, Ten Harkel AD, van Domburg RT, Spijkerboer AW, Utens EM, Bogers AJ, Helbing WA (2008) Follow-up outcomes 10 years after arterial switch operation for transposition of the great arteries: comparison of cardiological health status and health-related quality of life to those of the a normal reference population. Eur J Pediatr 167(9):995–1004. https://doi.org/10.1007/s00431-007-0626-5
Fredriksen PM, Pettersen E, Thaulow E (2009) Declining aerobic capacity of patients with arterial and atrial switch procedures. Pediatr Cardiol 30(2):166–171. https://doi.org/10.1007/s00246-008-9291-3
Giardini A, Khambadkone S, Rizzo N, Riley G, Pace Napoleone C, Muthialu N, Picchio FM, Derrick G (2009) Determinants of exercise capacity after arterial switch operation for transposition of the great arteries. Am J Cardiol 104(7):1007–1012. https://doi.org/10.1016/j.amjcard.2009.05.046
Kuebler JD, Chen MH, Alexander ME, Rhodes J (2016) Exercise performance in patients with D-loop transposition of the great arteries after arterial switch operation: long-term outcomes and longitudinal assessment. Pediatr Cardiol 37(2):283–289. https://doi.org/10.1007/s00246-015-1275-5
Samos F, Fuenmayor G, Hossri C, Elias P, Ponce L, Souza R, Jatene I (2016) Exercise capacity long-term after arterial switch operation for transposition of the great arteries. Congenit Heart Dis 11(2):155–159. https://doi.org/10.1111/chd.12303
van Beek E, Binkhorst M, de Hoog M, de Groot P, van Dijk A, Schokking M, Hopman M (2010) Exercise performance and activity level in children with transposition of the great arteries treated by the arterial switch operation. Am J Cardiol 105(3):398–403. https://doi.org/10.1016/j.amjcard.2009.09.048
Baggen VJ, Driessen MM, Meijboom FJ, Sieswerda GT, Jansen NJ, van Wijk SW, Doevendans PA, Leiner T, Schoof PH, Takken T, Breur JM (2015) Main pulmonary artery area limits exercise capacity in patients long-term after arterial switch operation. J Thorac Cardiovasc Surg 150(4):918–925. https://doi.org/10.1016/j.jtcvs.2015.07.101
Giardini A, Khambadkone S, Taylor A, Derrick G (2010) Effect of abnormal pulmonary flow distribution on ventilatory efficiency and exercise capacity after arterial switch operation for transposition of great arteries. Am J Cardiol 106(7):1023–1028. https://doi.org/10.1016/j.amjcard.2010.05.035
American Thoracic S, American College of Chest P (2003) ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 167(2):211–277. https://doi.org/10.1164/rccm.167.2.211
Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N, Nishibata K (1996) Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol 28(6):1567–1572
Bongers BC, Hulzebos EH, Helbing WA, Ten Harkel A, van Brussel M, Takken T (2016) Response profiles of oxygen uptake efficiency during exercise in healthy children. Eur J Prev Cardiol 23(8):865–873. https://doi.org/10.1177/2047487315611769
Bongers BC, Hulzebos HJ, Blank AC, van Brussel M, Takken T (2011) The oxygen uptake efficiency slope in children with congenital heart disease: construct and group validity. Eur J Cardiovasc Prev Rehabil 18(3):384–392. https://doi.org/10.1177/1741826710389390
Hollenberg M, Tager IB (2000) Oxygen uptake efficiency slope: an index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J Am Coll Cardiol 36(1):194–201
Sun XG, Hansen JE, Stringer WW (2012) Oxygen uptake efficiency plateau: physiology and reference values. Eur J Appl Physiol 112(3):919–928. https://doi.org/10.1007/s00421-011-2030-0
Godfrey S (1974) Methods of measuring the response to exercise in children. In: Exercise testing in children: applications in health and disease. W.B Saunders Company Ltd, London
Ten Harkel A, Takken T (2011) Normal values for cardiopulmonary exercise testing in children. Eur J Cardiovasc Prev Rehabil 18(4):676–677. https://doi.org/10.1177/1741826711410517
Van de Poppe DJ, Hulzebos E, Takken T, Low-Land Fitness Registry Study g (2019) Reference values for maximum work rate in apparently healthy Dutch/Flemish adults: data from the LowLands fitness registry. Acta Cardiol 74(3):223–230. https://doi.org/10.1080/00015385.2018.1478763
Mylius CF, Krijnen WP, van der Schans CP, Takken T (2019) Peak oxygen uptake reference values for cycle ergometry for the healthy Dutch population: data from the LowLands Fitness Registry. ERJ Open Res 5(2):00056–02018. https://doi.org/10.1183/23120541.00056-2018
Schutte NM, Nederend I, Hudziak JJ, Bartels M, de Geus EJ (2016) Twin-sibling study and meta-analysis on the heritability of maximal oxygen consumption. Physiol Genomics 48(3):210–219. https://doi.org/10.1152/physiolgenomics.00117.2015
Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR Jr, Schmitz KH, Emplaincourt PO, Jacobs DR Jr, Leon AS (2000) Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32(9 Suppl):S498-504
Klitsie LM, Roest AA, van der Hulst AE, Stijnen T, Blom NA, Ten Harkel AD (2013) Assessment of intraventricular time differences in healthy children using two-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr 26(6):629–639. https://doi.org/10.1016/j.echo.2013.03.006
Levy PT, Sanchez Mejia AA, Machefsky A, Fowler S, Holland MR, Singh GK (2014) Normal ranges of right ventricular systolic and diastolic strain measures in children: a systematic review and meta-analysis. J Am Soc Echocardiogr 27(5):549-560.e3. https://doi.org/10.1016/j.echo.2014.01.015
Baba R, Tsuyuki K, Kimura Y, Ninomiya K, Aihara M, Ebine K, Tauchi N, Nishibata K, Nagashima M (1999) Oxygen uptake efficiency slope as a useful measure of cardiorespiratory functional reserve in adult cardiac patients. Eur J Appl Physiol Occup Physiol 80(5):397–401. https://doi.org/10.1007/s004210050610
Van Laethem C, Bartunek J, Goethals M, Nellens P, Andries E, Vanderheyden M (2005) Oxygen uptake efficiency slope, a new submaximal parameter in evaluating exercise capacity in chronic heart failure patients. Am Heart J 149(1):175–180. https://doi.org/10.1016/j.ahj.2004.07.004
Singh TP, Rhodes J, Gauvreau K (2008) Determinants of heart rate recovery following exercise in children. Med Sci Sports Exerc 40(4):601–605. https://doi.org/10.1249/MSS.0b013e3181621ec4
Kondo C, Nakazawa M, Momma K, Kusakabe K (1998) Sympathetic denervation and reinnervation after arterial switch operation for complete transposition. Circulation 97(24):2414–2419
Crisafulli A, Piras F, Chiappori P, Vitelli S, Caria MA, Lobina A, Milia R, Tocco F, Concu A, Melis F (2007) Estimating stroke volume from oxygen pulse during exercise. Physiol Meas 28(10):1201–1212. https://doi.org/10.1088/0967-3334/28/10/006
Levy PT, Machefsky A, Sanchez AA, Patel MD, Rogal S, Fowler S, Yaeger L, Hardi A, Holland MR, Hamvas A, Singh GK (2016) Reference ranges of left ventricular strain measures by two-dimensional speckle-tracking echocardiography in children: a systematic review and meta-analysis. J Am Soc Echocardiogr 29(3):209-225.e6. https://doi.org/10.1016/j.echo.2015.11.016
Di Salvo G, Al Bulbul Z, Issa Z, Fadel B, Al-Sehly A, Pergola V, Al Halees Z, Al Fayyadh M (2016) Left ventricular mechanics after arterial switch operation: a speckle-tracking echocardiography study. J Cardiovasc Med (Hagerstown) 17(3):217–224. https://doi.org/10.2459/JCM.0000000000000316
Pettersen E, Fredriksen PM, Urheim S, Thaulow E, Smith HJ, Smevik B, Smiseth O, Andersen K (2009) Ventricular function in patients with transposition of the great arteries operated with arterial switch. Am J Cardiol 104(4):583–589. https://doi.org/10.1016/j.amjcard.2009.04.029
Massin MM, Hovels-Gurich HH, Gerard P, Seghaye MC (2006) Physical activity patterns of children after neonatal arterial switch operation. Ann Thorac Surg 81(2):665–670. https://doi.org/10.1016/j.athoracsur.2005.07.034
Duppen N, Takken T, Hopman MT, ten Harkel AD, Dulfer K, Utens EM, Helbing WA (2013) Systematic review of the effects of physical exercise training programmes in children and young adults with congenital heart disease. Int J Cardiol 168(3):1779–1787. https://doi.org/10.1016/j.ijcard.2013.05.086
Acknowledgements
The statistical advice of Dr N Van Geloven is highly appreciated.
Funding
The Dutch Heart Foundation in The Netherlands supported this research (Grant Number 2014T087 to van der Palen).
Author information
Authors and Affiliations
Contributions
CT: Conceptualization, Methodology, Formal analysis, Investigation, Writing—Original Draft, Visualization. RLFP: Conceptualization, Methodology, Data Curation, Investigation, Writing—Original Draft, Visualization. MGH: Writing- Reviewing and Editing. LR: Writing- Reviewing and Editing. MRMJ: Writing- Reviewing and Editing. NAB: Conceptualization, Validation, Writing- Reviewing and Editing. ADJH: Conceptualization, Methodology, Validation, Supervision, Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institutional review board. Written informed consent was obtained from all participants and/or their parents or legal guardians as appropriate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Terol Espinosa de los Monteros, C., Van der Palen, R.L.F., Hazekamp, M.G. et al. Oxygen Uptake Efficiency Slope is Strongly Correlated to VO2peak Long-Term After Arterial Switch Operation. Pediatr Cardiol 42, 866–874 (2021). https://doi.org/10.1007/s00246-021-02554-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-021-02554-9