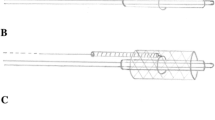
Abstract
Balloon-expandable stents, implanted in infants and children with congenital heart disease (CHD), often require redilation to match somatic growth. Small diameter stents may eventually require longitudinal surgical transection to prevent iatrogenic vascular stenosis. Intentional transcatheter stent fracture (TSF) is an emerging alternative approach to stent transection, but little is known about the optimal stent substrate and best protocol to improve the likelihood of successful TSF. Bench testing was performed with a stent dilation protocol. After recording baseline characteristics, stents were serially or directly dilated using ultra-high-pressure balloons (UHPB) until fracture occurred or further stent dilation was not possible. Stent characteristics recorded were as follows: cell design, metallurgy, mechanism, and uniformity of fracture. Stents tested included bare-metal coronary stents, premounted small diameter stents, and ePTFE-covered small diameter premounted stents. Ninety-four stents representing 9 distinct models were maximally dilated, with 80 (85%) demonstrating evidence of fracture. Comprehensive fracture details were recorded in 64 stents: linear and complete in 34/64 stents (53.1%), linear and incomplete in 9/64 stents (14.1%), transverse/complex and complete in 6/64 stents (9.4%), and transverse/complex and incomplete in 15/64 stents (23.4%). Stent fracture was not accomplished in some stent models secondary to significant shortening, i.e., “napkin-ring” formation. Serial dilation resulted in evidence of fracture in 62/67 (92.5%) stents compared with 18/27 (66.7%) stents in the direct dilation group (p = 0.003). Intentional TSF is feasible in an ex vivo model. Serial dilation more reliably expanded the stent and allowed for ultimate stent fracture, whereas direct large diameter dilation of stents was more likely to generate a “napkin-ring” configuration, which may be more resistant to fracture. In vivo animal and human testing is necessary to better understand the response to attempted TSF for newly developed stents as well as those currently in use.


Similar content being viewed by others
References
Mullins CE, O’Laughlin MP, Vick GW 3rd, Mayer DC, Myers TJ, Kearney DL, Schatz RA, Palmaz JC (1988) Implantation of balloon-expandable intravascular grafts by catheterization in pulmonary arteries and systemic veins. Circulation 77(1):188–199
Hascoet S, Baruteau A, Jalal Z, Mauri L, Acar P, Elbaz M, Boudjemline Y, Fraisse A (2014) Stents in paediatric and adult congenital interventional cardiac catheterization. Arch Cardiovasc Dis 107(8–9):462–475
Quandt D, Ramchandani B, Bhole V, Penford G, Mehta C, Dhillon R, Stumper O (2015) Initial experience with the cook formula balloon expandable stent in congenital heart disease. Catheter Cardiovasc Interv 85(2):259–266
Butera G, Giugno L, Basile D, Piazza L, Chessa M, Carminati M (2015) The Edwards Valeo lifestents in the treatment and palliation of congenital heart disease in infants and small children. Catheter Cardiovasc Interv 3:432–437
Stanfill R, Nykanen DG, Osorio S, Whalen R, Burke RP, Zahn EM (2008) Stent implantation is effective treatment of vascular stenosis in young infants with congenital heart disease: acute implantation and long-term follow-up results. Catheter Cardiovasc Interv 71(6):831–841
Ashwath R, Gruenstein D, Siwik E (2008) Percutaneous stent placement in children weighing less than 10 kilograms. Pediatr Cardiol 29(3):562–567
Maglione J, Bergersen L, Lock JE, McElhinney DB (2009) Ultra-high-pressure balloon angioplasty for treatment of resistant stenoses within or adjacent to previously implanted pulmonary arterial stents. Circ Cardiovasc Interv 2(1):52–58
Morray BH et al (2016) Intentional fracture of maximally dilated balloon-expandable pulmonary artery stents using ultra-high-pressure balloon angioplasty: a preliminary analysis. Circ Cardiovasc Interv 9(4):e003281
McElhinney DB, Marshall A, Schievano S (2013) Fracture of Cardiovascular Stents in Patients With Congenital Heart Disease: Theoretical and Empirical Considerations. Circ Cardiovasc Interv 6:575–585
Nordmeyer J, Khambadkone S, Coats L, Schievano S, Lurz P, Parenzan G, Taylor AM, Lock JE, Bonhoeffer P (2007) Risk stratification, systematic classification, and anticipatory management strategies for stent fracture after percutaneous pulmonary valve implantation. Circulation 115:1392–1397
Breinholt JP, Nugent A, Law MA, Justinoe H, Mullins CE, Ing FF (2008) Stent fractures in congenital heart disease. Catheter Cardiov Interv 72:977–982
Adlakha S, Sheikh M, Wu J, Burket MW, Pandya U, Colyer W, Eltahawy E, Cooper CJ (2010) Stent fracture in the coronary and peripheral arteries. J Interv Cardiol 23:411–419
Grogan JA, Leen SB, McHugh PE (2012) Comparing coronary stent material performance on a common geometric platform through simulated bench testing. J Mech Behav Biomed Mater 12:129–138
Kreutzer J, Rome JJ (2002) Open-cell design stents in congenital heart disease: a comparison of IntraStent vs Palmaz stents. Catheter Cardiovasc Interv 56(3):400–409
Ing FF, Grifka RG, Nihill MR, Mullins CE (1995) Repeat dilation of intravascular stents in congenital heart defects. Circulation 92(4):893–897
Morrow WR, Palmaz JC, Tio FO, Ehler WJ, VanDellen AF, Mullins CE (1993) Re-expansion of balloon-expandable stents after growth. J Am Coll Cardiol 22(7):2007–2013
Sathanandam SK, Haddad L, Subramanian S, Wright D, Philip R, Waller BR (2015) Unzipping of small diameter stents: an in vitro study. Catheter Cardiov Interv 85:249–258
Patel ND et al (2014) Single-center outcome analysis comparing reintervention rates of surgical arterioplasty with stenting for branch pulmonary artery stenosis in a pediatric population. Pediatr Cardiol 35(3):419–422
Bratincsak A, Moore JW, Gulker B, Choules B, Koren L, El-Said HG (2015) Breaking the limit: mechanical characterization of overexpanded balloon expandable stents used in congenital heart disease. Congenit Heart Dis 10(1):51–63
Ewert P, Riesenkampff E, Neuss M, Kretschmar O, Nagdyman N, Lange PE (2004) Novel growth stent for the permanent treatment of vessel stenosis in growing children: an experimental study. Catheter Cardiovasc Interv 62(4):506–510
Veeram Reddy SR, Welch TR, Wang J, Richardson JA, Forbess JM, Riegel M, Nugent AW (2015) A novel design biodegradable stent for use in congenital heart disease: mid-term results in rabbit descending aorta. Catheter Cardiovasc Interv 85(4):629–639
Acknowledgements
The authors would like to thank the following individuals (in alphabetical order) who assisted in testing and recording the stents described in this study: Anas Abu-Hazeem, MD, Osamah Aldoss, MD, Sarosh Batlivala, MD, Darren Berman MD, Tacy Downing, MD, Noa Holoshitz, MD, Asra Khan, MD, John Lozier, MD, Gira Morchi, MD, Joshua Murphy, MD, Suren Reddy, MD, Shyam Sathanandam, MD, Michael Seckeler, MD, Nathan Taggart, MD, Mariel Turner, MD, Wendy Whiteside, MD, Derek Williams, DO, and Matthew Zussman, MD. The authors would also like to thank Merit Medical for providing inflation devices, Bard Vascular for providing balloons, and Cook Medical and Bard for providing stents.
Author information
Authors and Affiliations
Contributions
MAC—involved in project creation, stent dilation session organization, data collection, manuscript preparation, and manuscript editing. GM—involved in stent dilation session, data collection, manuscript preparation, and manuscript editing. SD—involved in project creation, stent dilation session organization, data collection, manuscript preparation, and manuscript editing. RGG—involved in stent dilation session, data collection, manuscript preparation, and manuscript editing. DHG—involved in stent dilation session, data collection, manuscript preparation, and manuscript editing. BMG—involved in project creation, stent dilation session organization, data collection, manuscript preparation, and manuscript editing. BHG—involved in stent dilation session, data collection, manuscript preparation, and manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Pediatric Interventional Cardiology Early Career Society (PICES).
Appendix
Appendix
Methods (Serial Dilation)
Baseline characteristics of each stent (length, outer diameter at points A [proximal stent], B [mid stent], and C [distal stent]) were recorded with electronic digital calipers (SE, China) to the hundredth decimal place. The balloon was inflated to nominal pressure [Merit inflation device (Merit Medical Systems, South Jordan, Utah, United States)], and stent measurements A, B, and C were repeated. Balloons were deflated and stent measurements A, B, C and length were repeated. The balloon was reinflated to nominal pressure and measurements were taken at the corresponding points A, B, and C of the balloon.
Stents were remounted onto a balloon as close to the same length of the stent as possible at each redilation to minimize stent foreshortening. Stents were remounted on an ultra-high-pressure balloon (UHPB) Dorado, Conquest, or Atlas (BARD Peripheral Vascular, Inc, Tempe, Arizona), inflated to the rated burst pressure, and stent measurements A, B, and C were re-recorded. The balloon was deflated and stent measurements at points A, B, C and length were repeated. The UHPB was reinflated to rated burst pressure and points A, B, and C were remeasured. If any fracture occurred, the mechanism of fracture (longitudinal, circumferential, complex) and atmospheres required were recorded. If no fractures occurred, the stent was remounted onto a larger UHPB whose size was determined by the type of stent, as described below. Measurements were repeated as per the above outlined protocol until fracture occurred or further stent dilatation was not possible (“napkin-ring” formation occurred).
After initial expansion, coronary stents were remounted onto 6-mm UHPB, which were then increased by 2-mm increments until fracture occurred or further stent dilation was not possible. Premounted Genesis, Palmaz Blue (Cordis, Miami, FL), and Cook Formula series (Cook Medical Inc., Bloomington, IN) were dilated as follows: 4- to 5-mm-diameter stents were placed on 6-mm UHPB, 6- to 7-mm-diameter stents were placed on 8-mm UHPB, and 8- to 9-mm-diameter stents were placed on 10-mm UHPB. These stents were further dilated by UHPB of increasing 2-mm increments until fracture occurred or further stent dilation was not possible.
Valeo (BARD Peripheral) 6- to 8-mm-diameter premounted biliary stents were initially redilated on a 10-mm UHPB. All stents (6–10 mm diameter premounted) were then remounted onto a 14-mm UHPB, followed by 18-mm UHPB, and then increasing by 2-mm UHPB increments until fracture occurred or further stent dilation was not possible. Atrium iCast covered stents (Atrium Maquet Getinge Group, Hudson, NH) were redilated as outlined above for other stents based on the initial balloon the stent was mounted on.
Methods (Direct Dilation)
Baseline characteristics of each stent (length, outer diameter at points A [proximal stent], B [mid stent], and C [distal stent]) were recorded with electronic digital calipers (SE, China) to the hundredth decimal place. The balloon was inflated to nominal pressure [Merit inflation device (Merit Medical Systems, South Jordan, Utah, United States)], and stent measurements A, B, and C were repeated. Balloons were deflated and stent measurements A, B, C and length were repeated. The balloon was reinflated to nominal pressure and measurements were taken at the corresponding points A, B, and C of the balloon.
Stents were remounted onto a balloon as close to the same length of the stent as possible at each redilation to minimize stent foreshortening. Stents were remounted on an ultra-high-pressure balloon (UHPB) Dorado, Conquest, or Atlas (BARD Peripheral Vascular, Inc, Tempe, Arizona) selected to match the diameter of the largest serial diameter. The balloon was inflated to rated burst pressure, and stent measurements A, B, and C were re-recorded. The balloon was deflated and stent measurements at points A, B, C and length were repeated. The UHPB was reinflated to rated burst pressure and points A, B, and C were remeasured. If any fracture occurred, the mechanism of fracture (longitudinal, circumferential, complex) and atmospheres required were recorded. If no fractures occurred, the stent was remounted onto a larger UHPB whose size was determined by the type of stent, as described below. Measurements were repeated as per the above outlined protocol until fracture occurred or further stent dilatation was not possible (“napkin-ring” formation occurred).
After initial expansion, premounted Genesis, Palmaz Blue (Cordis, Miami, FL), and Cook Formula series (Cook Medical Inc., Bloomington, IN) were dilated as follows: 4- to 5-mm-diameter stents were placed on 10-mm UHPB, 6- to 7-mm-diameter stents were placed on 14-mm UHPB, and 8- to 9-mm-diameter stents were placed on 18-mm UHPB. These stents were further dilated by UHPB of increasing 2-mm increments until fracture occurred or further stent dilation was not possible.
Valeo (BARD Peripheral) 6- to 8-mm-diameter premounted biliary stents were initially redilated on a 16-mm UHPB, 9- to 10-mm-diameter premounted stents were placed on 20-mm UHPB and then increasing by 2-mm UHPB increments until fracture occurred or further stent dilation was not possible. Atrium iCast covered stents (Atrium Maquet Getinge Group, Hudson, NH) were redilated as outlined above for other stents based on the initial balloon the stent was mounted on.
Rights and permissions
About this article
Cite this article
Crystal, M.A., Morgan, G.J., Danon, S. et al. Serial Versus Direct Dilation of Small Diameter Stents Results in a More Predictable and Complete Intentional Transcatheter Stent Fracture: A PICES Bench Testing Study. Pediatr Cardiol 39, 120–128 (2018). https://doi.org/10.1007/s00246-017-1736-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-017-1736-0