Abstract
Myocardial contractility and relaxation are highly dependent on calcium homeostasis. Immature myocardium, as in pediatric patients, is thought to be more dependent on extracellular calcium for optimal function. For this reason, intravenous calcium chloride infusions may improve myocardial function in the pediatric patient. The objectives of this study were to report the hemodynamic changes seen after administration of continuous calcium chloride to critically ill children. We retrospectively identified pediatric patients (newborn to 17 years old) with hemodynamic instability admitted to the cardiac ICU between May 2011 and May 2012 who received a continuous infusion of calcium chloride. The primary outcome was improvement in cardiac output, assessed by arterial-mixed venous oxygen saturation (A–V) difference. Sixty-eight patients, mean age 0.87 ± 2.67 years, received a total of 116 calcium infusions. Calcium chloride infusions resulted in significant improvements in primary and secondary measures of cardiac output at 2 and 6 h. Six hours after calcium initiation, A–V oxygen saturation difference decreased by 7.4 % (32.6 ± 2.1 to 25.2 ± 2.0 %, p < 0.001), rSO2 increased by 5.5 % (63.1 vs 68.6 %, p < 0.001), and serum lactate decreased by 0.9 mmol/l (3.3 vs 2.4 mmol/l, p < 0.001) with no change in HR (149.1 vs 145.6 bpm p = 0.07). Urine output increased 0.66 ml/kg/h in the 8-h period after calcium initiation when compared to pre-initiation (p = 0.003). Neonates had the strongest evidence of effectiveness with other age groups trending toward significance. Calcium chloride infusions improve markers of cardiac output in a heterogenous group of pediatric patients in a cardiac ICU. Neonates appear to derive the most benefit from utilization of these infusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calcium homeostasis is crucial for effective myocardial performance [19]. In particular, the regulation of intracellular cardiomyocyte calcium concentration is necessary for efficient myocardial contractility and relaxation. In mature mammalian myocytes, the primary source of calcium is the sarcoplasmic reticulum (SR) [26, 29], while in immature myocytes the SR is structurally and functionally underdeveloped so the relative contribution of calcium influx across the cell membrane (sarcolemma) is thought to play a much larger role [15, 20, 25]. Therefore, the immature myocardium is thought to be more dependent on extracellular calcium for optimal function. For this reason, we believe that intravenous calcium chloride infusions improve myocardial function in the pediatric patient with low cardiac output (LCO).
To date, no published literature exists supporting the use of calcium chloride infusions to improve hemodynamics in patients with LCO. Levosimendan, a calcium sensitizer and inodilator, is the only medication that may be slightly similar mechanistically, although it does not alter extracellular calcium concentrations. Levosimendan is currently in a Phase 3 adult trial for use in the USA for the prevention of LCO following cardiac surgery. Levosimendan enhances myocardial contractility without increasing oxygen consumption by sensitizing the myofilament to calcium, and altering calcium homeostasis. Clinical trials suggest that levosimendan is effective as an inotropic agent and vasodilator, supporting the concept that altering calcium homeostasis can improve cardiovascular hemodynamics in adults [3, 5, 10, 11, 16, 22, 28] and children [4, 8, 12, 14, 18, 24]. However, while this suggests a theoretical hemodynamic benefit of calcium infusions, there is concern that hypercalcemia-associated adverse events such as pancreatitis, nephrolithiasis, and renal dysfunction may be more prevalent with this treatment modality [33].
Despite the paucity of published data regarding the use as a continuous calcium infusion, and because of the theoretical benefits and undefined adverse event profile, calcium chloride infusions have been used as an “inotrope” for children with hemodynamic compromise for many years. The aim of this study was to characterize the hemodynamic effects of calcium chloride infusions in pediatric patients with LCO and to establish the foundation for a future study. We hypothesized that calcium chloride infusions improve noninvasive, surrogate markers of cardiac output in this population.
Materials and Methods
Patients
The Institutional Review Board at Cincinnati Children’s Hospital Medical Center approved this study. A retrospective chart review was performed in children aged 0–17 years admitted to the cardiac intensive care unit (CICU) at CCHMC from May 2011–May 2012, who received a calcium chloride infusion for the indication of LCO. The diagnosis of LCO was made by the CICU team using an accepted set of clinical (tachycardia, oliguria, poor perfusion) and/or laboratory (widened arterial-mixed venous oxygen saturation difference, lactic acidosis) indicators [9]. The infusion was started at 5–10 mg/kg/h. LCO was classified as surgical if it occurred within 1 week of cardiopulmonary bypass for palliation/repair of congenital heart disease (CHD), or non-surgical for all other patients. Patients were divided into those with univentricular and biventricular physiologies for subgroup analysis. Patients were included multiple times if they were treated with a calcium chloride infusion on more than one occasion because the effects of calcium chloride infusions quickly dissipate with discontinuation. Patients who died before the study data were obtained, and those treated with mechanical circulatory support during the infusion were excluded.
Medical records were reviewed to retrieve primary diagnosis, age, and weight. For subgroup analysis, patients were divided into neonate (0–30 days), infant (30–180 days), and children (>180 days). Vital signs were monitored in the CICU and were extracted from the electronic medical record. Despite this being a retrospective study, laboratory blood draws were done at specifically timed intervals because the calcium chloride infusions were ordered via a pre-defined order set in the electronic order entry system. Markers of hemodynamic status were collected at baseline and approximately 2 and 6 h after infusion initiation. Hemodynamic assessment included: heart rate (HR), blood pressure (BP), systemic arterial oxygen saturation (via pulse oximetry); mixed venous oxygen saturation (obtained from an upper extremity central venous catheter with the tip in the superior vena cava); cerebral regional oxygen saturation (rSO2) obtained by near-infrared spectroscopy; serum lactate; and dosages of other inotrope infusions (epinephrine, milrinone, and/or vasopressin) to calculate a vasoactive infusion score (VIS). We chose to use coefficients for milrinone, vasopressin, and epinephrine that would convert them to an integer value and to give each medication equal weight in the calculation as originally done by Wernovsky and colleagues and then modified by Gaies and colleagues [6, 32]. In their paper, Gaies and colleagues consider patients in groups defined in five unit increments of the VIS, so in our analysis we defined a significant change from baseline as a change in the VIS of greater than 5 [6].
To assess end organ perfusion, cumulative urine output in the 8-h period before and after (to facilitate data collection) calcium initiation was recorded. The arterial-mixed venous oxygen saturation (A–V) difference was calculated by subtracting mixed venous oxygen saturation from the systemic arterial oxygen saturation, with a lower numerical value indicating better cardiac output. Line placement for measurement of mixed venous oxygen saturation was confirmed by radiograph.
Biochemical markers of pancreatitis (amylase and lipase) were initially collected daily then less frequently if they remained in the normal range. We defined an elevation in pancreatic enzymes as change in one or both from normal to abnormal (amylase >105 units/l; lipase >231 units/l) or an increase in greater than 50 % from baseline. Serum ionized calcium concentration (the physiologically active, nonprotein bound portion of extracellular calcium) was recorded at baseline and daily for duration of therapy and was defined as low (<1.0 mmol/l), normal (1–1.45 mmol/l), and high (>1.45 mmol/l).
Statistics
Patient data are reported as mean (SD) and median (IQR) for continuous variables and frequency (percent) for categorical variables. To assess the effect of calcium infusion over time, change from baseline scores were calculated for each infusion at 2 and 6 h after calcium initiation for the primary outcome variable, A–V saturation difference, and the secondary outcome variables: changes in HR, systolic BP, diastolic BP, mean BP, lactate, urine output, and cerebral rSO2. Change from baseline scores versus the hypothesized value of zero at each of the post-baseline measurement time points were tested (i.e., 2, 6 h) using a repeated-measures method to account for multiple infusions for the same patient. Similar methodology was used to test change from baseline scores versus the hypothesized value of zero, separated by age group (neonates, infants, and children), etiology (non-surgical and surgical), and ventricular anatomy (univentricular and biventricular). Between-group testing of the scores was also performed. For each subgroup analysis, only the first two infusions were included for patients with multiple infusions. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC) with a p value of <0.05 considered significant.
Results
Patient Demographics/Characteristics at the Time of Infusion Initiation
Sixty-eight patients with LCO received a total of 116 calcium infusions: 65 (56 %) were in neonates, 21 (18 %) were in infants, and 30 (26 %) were in children. The etiology of LCO was non-surgical in 55 (47 %) cases and surgical in 61 (52 %) cases. There were slightly more cases with biventricular physiology 68/116 (59 %) compared with univentricular physiology 48/116 (41 %). A majority of the cases (80 %) in our study had normal baseline calcium levels.
The median duration for a calcium infusion was 67.25 h (IQR 37.33, 130.44) with most patients (83 %) being on the infusion for <7 days. The peak serum ionized calcium concentration over the course of the infusion was within normal range in approximately half of all patients. Patient characteristics are summarized in Table 1.
Hemodynamic Response to the Initiation of Calcium Chloride Infusions
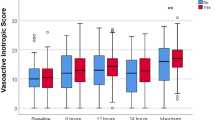
There was a significant improvement in A–V difference at 2 h (32.6 ± 2.1 to 29.1 ± 1.5 %, p = 0.02) and a more marked, clinically significant improvement at 6 h (32.6 ± 2.1 to 25.2 ± 2.0 %, p < 0.001) after initiation of a calcium chloride infusion. In addition, secondary variables of cardiac output and BP parameters all improved 2 and 6 h after calcium was begun with a non-statistically significant decrease in HR at both time points (Fig. 1). Two hours post-infusion, the cerebral rSO2 increased by 2.3 % (63.1 vs 65.4 %, p = 0.003) and serum lactate decreased by 0.4 mmol/l (3.3 vs 2.9 mmol/l, p = 0.008), while 6 h after cerebral rSO2 increased by 5.5 % (63.1 vs 68.6 %, p < 0.001) and serum lactate decreased by 0.9 mmol/l (3.3 vs 2.4 mmol/l, p < 0.001). End organ perfusion also improved, evident by a 29 % increase in urine output (2.28 ± 0.24 to 2.91 ± 0.26 ml/kg/h, p = 0.0003) in the 8-h period after calcium initiation compared to the 8-h period prior.
Calcium chloride efficacy for the entire cohort. bpm beats per minute, SBP systolic blood pressure, DBP diastolic blood pressure, MAP mean arterial pressure, AV arterial–venous, rSO2 regional oxygen saturation; all values are expressed as standard error; start (*) indicates statistical significance with a p value <0.05
In the 80 % of cases which had normal baseline calcium levels, there were significant improvements in A–V difference (2 h, p = 0.001; 6 h, p < 0.001); cerebral rSO2 (2 h, p = 0.02; 6 h, p < 0.001); and lactate (6 h, p < 0.001). There were no significant changes in primary or secondary outcome measures in cases with low or high baseline calcium levels. When calcium chloride was the only vasoactive medication (16/116, 14 %), the initiation of calcium led to a clinically relevant decrease in the A–V difference by 6.8 %, but this was not statistically significant. In these patients, there was also a significant improvement in lactate (p = 0.01) and cerebral rSO2 (p = 0.03).
Simultaneous inotrope use at the time of initiation of calcium chloride infusion was common, with 86 % of patients on additional inotropes when calcium was instituted. Notably, in the 2- and 6-h period following calcium infusion initiation, the VIS (measuring the change in vasopressor use and dosage) did not change significantly from baseline in 96 % of cases (96/100) Interventions that involved adding or altering the doses of other vasopressor medications did not occur in most circumstances. Only four patients had an increase in their VIS score of >5 after the initiation of the calcium chloride infusion. There were only two instances in which an additional vasoactive infusion was added that had not been prescribed prior to initiation of calcium.
Effect of Age on Hemodynamic Response
Neonates experienced improvements in primary and secondary measures of cardiac output primarily at 6 h. Absolute A–V difference decreased by 5.5 % (28.3 ± 2.8 to 22.8 ± 2.0 %, p = 0.05) and by 13 % (28 ± 3 to 15 ± 3 %, p = 0.01) at 2 and 6 h, respectively, after calcium initiation. At 6 h, lactate decreased by 0.9 mmol/l (4.1 ± 0.5 to 3.2 ± 0.3 mmol/l, p = 0. 01) and cerebral rSO2 increased by 5.5 % (65 ± 3 to 70 ± 1 %, p = 0.01). The infant and children cohorts had a modest clinical response, but none of the changes reached statistical significance (Table 2). Urine output increased in all three groups following calcium initiation but only reached statistical significance in neonates (2.3 ± 0.3 to 3.3 ± 0.4 ml/kg/h, p = 0.001).
Effect of Etiology of Low Cardiac Output and Ventricular Anatomy on Calcium Efficacy
Non-surgical and surgical patients both had improvements in primary and secondary outcome measures primarily at 6 h (Table 3). The only significant change at 2 h was an increase of 2.2 % (66.9 ± 3.3 to 69.1 ± 0.9 %, p = 0.03) in rSO2 in the surgical group. At 6 h, surgical patients also had a clinically significant decrease in A–V difference of 10.3 % (33.1 ± 3.6 to 23.1 ± 2.9 %, p = 0.04) and lactate of 0.9 mmol/l (3.7 ± 0.4 to 2.8 ± 0.3 mmol/l, F 0.01). Non-surgical patients had a decrease in lactate of 0.8 mmol/l (3.6 ± 0.6 to 2.8 ± 0.4 mmol/l, p = 0.04) and an increase in rSO2 of 9.0 % (65.0 ± 3.3 to 74.0 ± 2.5 %, p = 0.01).
When comparing patients with uni- versus biventricular physiology, only univentricular patients had significant improvement in A–V difference which decreased by 7 % at 2 h (25.5 ± 3.5 to 18.5 ± 2.3 %, p = 0.04) and 10.3 % at 6 h (25.5 ± 3.5 to 15.5 ± 3.4 %, p = 0.04). Univentricular patients also had decrease in lactate at 6 h (3.6 ± 0.6 to 2.5 ± 0.4 mmol/l, p = 0.01) as well as statistically significant improvements in all BP parameters. The 6-h decrease in A–V difference in patients with biventricular physiology was clinically significant (−7.4 ± 2.9 %) and trended toward statistical significance (p = 0.06). These patients had improvement in rSO2 at 2 h (66.2 ± 2.5 to 68.2 ± 0.9 %, p = 0.04) and 6 h (66.2 ± 2.5 to 71.0 ± 2.0 %, p = 0.03) as well as lactate at 6 h (3.7 ± 0.5 to 2.9 ± 0.3 %, p = 0.02). All other measures did not reach statistical significance (Table 3).
Complication Monitoring During Treatment
Screening for pancreatic enzyme elevation occurred daily for 22 % (26/116) of the infusions. Eleven percent (4/26) of the patients screened had elevation in amylase and/or lipase on a daily level after initiation of a calcium chloride infusion. There were no changes in patient management following the detection of elevated pancreatic enzymes.
Discussion
Although studied retrospectively, it appears that calcium chloride, administered as an infusion, improves markers of cardiac output in pediatric patients with hemodynamic compromise. Patients of all ages, particularly neonates, had a positive hemodynamic response after initiation of calcium chloride. In our heterogeneous population with various anatomical diagnoses and etiologies of LCO, calcium chloride infusions improved hemodynamics without a coincident increase in HR. The absence of a positive chronotropic effect is crucial, as calcium infusions improve patient status by increasing tissue oxygen delivery with minimal increase in HR, lessening the increased metabolic demand that is often seen with traditional inotropes (β-agonists) [1, 27, 31]. The mechanism by which calcium chloride improves patient stability may not be solely due to an inotropic effect but also secondary to arterial vasoconstriction; however, we could not clearly delineate these effects from our data. Our findings taken together suggest that calcium chloride may be considered in the treatment of neonates and potentially of infants and children with LCO.
Two time points for evaluation, 2- and 6-h post-infusion, were chosen in order to define the temporal effects of calcium. We found that by 2 h many hemodynamic parameters had improved but that the changes seen at 6 h were superior. This is unlike traditional vasoactive infusions, i.e., epinephrine, that notoriously have a quick onset but their effect plateaus and often necessitates further dose escalation. Interestingly, despite the slower onset of response, medical management in all age groups was rarely escalated after the initiation of the calcium chloride infusion. This is demonstrated by the fact that only a small percentage of patients (4 %) had a significant increase in the VIS (which should account for changes in the medication regimen) at either the 2- or 6-h time point following the initiation. Notably, a majority (58 %) of the patients were on either no vasoactive medications or only one vasoactive infusion prior to initiation of calcium chloride, leaving other infusion options available but not utilized by the primary team. Despite the fact that in most cases the vasoactive drugs were not altered, we were unable to account for other interventions that may have occurred. Examples of interventions that may have occurred and were not accounted for include administration of sedation medications, fluid bolus or packed red blood cells, manipulation of ventilator settings, or temperature regulation. Future prospective studies to define the true effects of calcium chloride infusions are needed and should be designed to control for other interventions that may occur simultaneously.
Historically, age and calcium levels were used to predict which patients would benefit from a calcium chloride infusion with neonates and those with low serum calcium levels thought to be most likely to benefit. In our study, as hypothesized, neonates had the most robust improvement in cardiac output. This response is supported by the fact that extracellular calcium is critical in patients with immature cardiomyocytes [15, 20, 25]. Despite the lack of statistical significance, there was a trend toward improvement in hemodynamics in infants and children as well and the use of calcium chloride infusions in these age groups should not be discounted. Using calcium chloride infusions in all age groups, which is now our practice, is not without precedent as adult and pediatric studies have suggested that levosimendan may improve hemodynamics regardless of patient age [2–5, 8, 10–12, 14, 16–18, 21–24, 28, 30]. In addition to broadening of the use of calcium chloride infusions across the age spectrum, we no longer limit their use to patients with low baseline calcium levels. Our data support this practice, as patients with normal baseline serum ionized calcium, 80 % of our cohort, showed a significant hemodynamic response to calcium chloride infusion initiation.
Although we hypothesized that patients with non-surgical etiologies of LCO may have a more robust response to calcium administration, we found an equally significant response in children who had undergone surgical repair/palliation for CHD and this finding cannot be explained a predominance of neonates in the surgical group. Interestingly, patients with univentricular physiology had a more pronounced BP response to calcium chloride as opposed to those with biventricular circulations. This is similar to the effect of calcium modification in patients with univentricular physiology that has been demonstrated by the positive outcomes with perioperative utilization of levosimendan [7]. The improvement in univentricular physiology is a critical finding, as medical options for these children are often limited [13].
Finally, of the patients screened for complications, no significant biochemical or clinical evidence of pancreatitis was found. Given the small number of patients screened for complications, we cannot state definitively that no complications occurred; however, the lack of alterations in practice suggests that widespread abnormalities were not common. It is also possible that with more thorough screening more cases of subclinical pancreatitis would be revealed. To this end, our institutional policy is to utilize a clinical order set in patients receiving calcium chloride infusions to standardize monitoring for all theoretical adverse events.
Study Limitations
This study has several limitations, many of them inherent in a retrospective study design. We were not able to collect complete data for all patients. Our data did not allow for transparency of simultaneous interventions, other than the additional initiation or escalation of other vasoactive infusions. While we collected data for three other inotropic infusions, including dosage, there are likely other confounding variables for which we do not account in medically complex patients. We also did not examine the effects of bolus calcium chloride on hemodynamics. There were many patients who were not screened for relevant side effects. Calcium infusion dosing was not standardized, and initiation doses varied from 5–10 mg/kg/h. Finally, data were not available to assess for ventricular tachyarrhythmias, one of the side effects that has been reported to occur during levosimendan infusion [5, 17, 21, 28].
Conclusion
The initiation of calcium chloride infusions in pediatric patients with LCO appears to be associated with improvement in hemodynamics and end organ perfusion. The improvement in hemodynamic status does not appear to be secondary to increased HR suggesting that clinical improvement was primarily due to a positive inotropic effect. Our data also suggest that calcium has beneficial effects among patients with normal baseline ionized calcium level, and its effect may be dependent on age but independent of etiology of LCO. Future prospective studies are required to delineate the hemodynamic effects of calcium chloride infusions as well as to define the adverse event profile associated with the therapy.
Abbreviations
- LCO:
-
Low cardiac output
- A–V:
-
Arterial-mixed venous oxygen saturation
- rSO2:
-
Regional oxygen saturation
- HR:
-
Heart rate
- BP:
-
Blood pressure
- VIS:
-
Vasoactive infusion score
- CHD:
-
Congenital heart disease
- CICU:
-
Cardiac intensive care unit
References
Barrington KJ, Finer NN, Chan WK (1995) A blind, randomized comparison of the circulatory effects of dopamine and epinephrine infusions in the newborn piglet during normoxia and hypoxia. Crit Care Med 23:740–748
Biegus J, Zymlinski R, Kulej K, Szachniewicz J, Banasiak W, Jankowska EA, Ponikowski P (2013) Application of levosimendan in acute heart failure patients with symptoms of low cardiac output: case series report. Kardiol Pol 71:275–278
Cleland JG, Freemantle N, Coletta AP, Clark AL (2006) Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROACTIVE. Eur J Heart Fail 8:105–110
Egan JR, Clarke AJ, Williams S, Cole AD, Ayer J, Jacobe S, Chard RB, Winlaw DS (2006) Levosimendan for low cardiac output: a pediatric experience. J Intensive Care Med 21:183–187
Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L, Steering C, Investigators of the Levosimendan Infusion versus Dobutamine S (2002) Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 360:196–202
Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, Charpie JR, Hirsch JC (2010) Vasoactive–inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass*. Pediatr Crit Care Med 11:234–238
Garisto C, Favia I, Ricci Z, Di Chiara L, Morelli S, Giorni C, Vitale V, Picardo S, Di Donato RM (2010) Initial single-center experience with levosimendan infusion for perioperative management of univentricular heart with ductal-dependent systemic circulation. World J Pediatr Congenit Heart Surg 1:292–299
Hoffman TM (2011) Newer inotropes in pediatric heart failure. J Cardiovasc Pharmacol 58:121–125
Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, Bailey JM, Akbary A, Kocsis JF, Kaczmarek R (2003) Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 107:996–1002
Husebye T, Eritsland J, Muller C, Sandvik L, Arnesen H, Seljeflot I, Mangschau A, Bjornerheim R, Andersen GO (2013) Levosimendan in acute heart failure following primary percutaneous coronary intervention-treated acute ST-elevation myocardial infarction. Results from the LEAF trial: a randomized, placebo-controlled study. Eur J Heart Fail 15:565–572
Kodalli RK, Sundar AS, Vakamudi M, Ravulapali H, Nandipati S, Chandrasekaran N, Karthekeyan RB (2013) Effect of levosimendan on hemodynamic changes in patients undergoing off-pump coronary artery bypass grafting: a randomized controlled study. Ann Card Anaesth 16:94–99
Lechner E, Hofer A, Leitner-Peneder G, Freynschlag R, Mair R, Weinzettel R, Rehak P, Gombotz H (2012) Levosimendan versus milrinone in neonates and infants after corrective open-heart surgery: a pilot study. Pediatr Crit Care Med 13:542–548
Li J, Zhang G, Holtby H, Humpl T, Caldarone CA, Van Arsdell GS, Redington AN (2006) Adverse effects of dopamine on systemic hemodynamic status and oxygen transport in neonates after the Norwood procedure. J Am Coll Cardiol 48:1859–1864
Magliola R, Moreno G, Vassallo JC, Landry LM, Althabe M, Balestrini M, Charroqui A, Salgado G, Lataza E, Chang AC (2009) Levosimendan, a new inotropic drug: experience in children with acute heart failure. Arch Argent Pediatr 107:139–145
Mahony L (1996) Regulation of intracellular calcium concentration in the developing heart. Cardiovasc Res 31:E61–E67
Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Poder P, Kivikko M, Investigators S (2007) Levosimendan versus dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA 297:1883–1891
Moiseyev V, Poder P, Andrejevs N, Ruda M, Golikov A, Lazebnik L, Kobalava Z, Lehtonen L, Laine T, Nieminen MS (2002) Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN). Eur Heart J 23:1422–1432
Momeni M, Rubay J, Matta A, Rennotte MT, Veyckemans F, Poncelet AJ, Clement de Clety S, Anslot C, Joomye R, Detaille T (2011) Levosimendan in congenital cardiac surgery: a randomized, double-blind clinical trial. J Cardiothorac Vasc Anesth 25:419–424
Morgan JP (1991) Abnormal intracellular modulation of calcium as a major cause of cardiac contractile dysfunction. N Engl J Med 325:625–632
Nassar R, Reedy M, Anderson P (1987) Developmental changes in the ultrastructure and sarcomere shortening of the isolated rabbit ventricular myocyte. Circ Res 61:465–483
Nieminen MS, Akkila J, Hasenfuss G, Kleber FX, Lehtonen LA, Mitrovic V, Nyquist O, Remme WJ (2000) Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol 36:1903–1912
Packer M, Colucci WS, Fisher L, Massie BM, Teerlink JR, Young J, Padley RJ, Thakkar R, Delgado-Herrera L, Salon J, Garratt C, Huang B, Sarapohja T (2013) Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail 1:103–111
Pellicer A, Riera J, Lopez-Ortego P, Bravo MC, Madero R, Perez-Rodriguez J, Labrandero C, Quero J, Buno A, Castro L, Lubomirov R, Cabanas F (2013) Phase 1 study of two inodilators in neonates undergoing cardiovascular surgery. Pediatr Res 73:95–103
Ricci Z, Garisto C, Favia I, Vitale V, Di Chiara L, Cogo PE (2012) Levosimendan infusion in newborns after corrective surgery for congenital heart disease: randomized controlled trial. Intensive Care Med 38:1198–1204
Seguchi M, Harding JA, Jarmakani JM (1986) Developmental change in the function of sarcoplasmic reticulum. J Mol Cell Cardiol 18:189–195
Sham J, Cleemann L, Morad M (1992) Gating of the cardiac Ca2+ release channel: the role of Na+ current and Na (+)-Ca2+ exchange. Science 255:850–853
Simaan JA, Fawaz G, Jabbour K (1988) Comparison of the cardiodynamic and metabolic effects of dobutamine with those of norepinephrine and dopamine in the dog isolated heart. Naunyn-Schmiedebergs Arch Pharmacol 338:174–179
Slawsky MT, Colucci WS, Gottlieb SS, Greenberg BH, Haeusslein E, Hare J, Hutchins S, Leier CV, LeJemtel TH, Loh E (2000) Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Circulation 102:2222–2227
Stern MD (1992) Theory of excitation-contraction coupling in cardiac muscle. Biophys J 63:497–517
Tuomainen PO, Magga J, Timonen P, Miettinen K, Kurttila M, Vanninen E, Laitinen T, Timonen K, Punnonen K, Parviainen I, Uusaro A, Vuolteenaho O, Kivikko M, Peuhkurinen K (2013) Intermittent levosimendan treatment in patients with severe congestive heart failure. Clin Res Cardiol 102:485–493
Vasu MA, O’Keefe DD, Kapellakis GZ, Vezeridis MP, Jacobs ML, Daggett WM, Powell W (1978) Myocardial oxygen consumption: effects of epinephrine, isoproterenol, dopamine, norepinephrine, and dobutamine. Am J Physiol Heart Circ Physiol 235:H237–H241
Wernovsky G, Wypij D, Jonas RA, Mayer JE, Hanley FL, Hickey PR, Walsh AZ, Chang AC, Castañeda AR, Newburger JW (1995) Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 92:2226–2235
Williams RH, Larsen PR (2003) Williams textbook of endocrinology, 10th edn. Saunders, Philadelphia
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Averin, K., Villa, C., Krawczeski, C.D. et al. Initial Observations of the Effects of Calcium Chloride Infusions in Pediatric Patients with Low Cardiac Output. Pediatr Cardiol 37, 610–617 (2016). https://doi.org/10.1007/s00246-015-1322-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-015-1322-2