Abstract
This study aimed to evaluate the time course of perioperative blood glucose levels of children undergoing cardiac surgery for congenital heart disease in relation to endogenous stress hormones, inflammatory mediators, and exogenous factors such as caloric intake and glucocorticoid use. The study prospectively included 49 children undergoing cardiac surgery. Blood glucose levels, hormonal alterations, and inflammatory responses were investigated before and at the end of surgery, then 12 and 24 h afterward. In general, blood glucose levels were highest at the end of surgery. Hyperglycemia, defined as a glucose level higher than 8.3 mmol/l (>150 mg/dl) was present in 52% of the children at the end of surgery. Spontaneous normalization of blood glucose occurred in 94% of the children within 24 h. During surgery, glucocorticoids were administered to 65% of the children, and this was the main factor associated with hyperglycemia at the end of surgery (determined by univariate analysis of variance). Hyperglycemia disappeared spontaneously without insulin therapy after 12–24 h for the majority of the children. Postoperative morbidity was low in the study group, so the presumed positive effects of glucocorticoids seemed to outweigh the adverse effects of iatrogenic hyperglycemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hyperglycemia is a regular phenomenon in critically ill children after surgical repair or palliation of congenital heart defects. Some recent studies have shown an association of hyperglycemia with increased postoperative morbidity and mortality in these children [7, 18, 35].
The adult literature contains a debate on the usefulness of intensive insulin therapy for glucose control to improve morbidity and mortality rates for cardiac surgical patients [8, 13]. The only randomized controlled study of critically ill children showed improved short-term outcome after treatment with intensive insulin therapy targeting blood glucose levels to age-adjusted normal fasting concentrations [31], but debate exists on the harm of insulin-induced hypoglycemic events [11].
For glucose control protocols to be most efficient, they should be based on pathophysiologic mechanisms [30]. Several studies have addressed this topic for critically ill adults [15, 16, 26], but such studies of critically ill children are lacking.
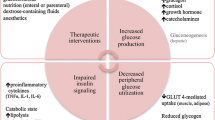
Hyperglycemia in critically ill children is caused by multiple factors, among which endogenous stress hormones [2], inflammatory mediators, oxidative stress, and therapeutic interventions such as glucose and drug administration are the main causative factors.
Children undergoing cardiopulmonary bypass (CPB) surgery often receive perioperative glucocorticoids to attenuate the systematic inflammatory response, but to date, no clinical benefit has been shown [22]. However, hyperglycemia is a well-known side effect of glucocorticoid use. We hypothesized that in some settings, the adverse effects of steroid-induced hyperglycemia could outweigh the anticipated benefits.
The current study aimed to evaluate blood glucose levels in children undergoing open heart surgery relative to stress-induced endogenous hormonal production, inflammatory mediators, and exogenous factors such as caloric intake and glucocorticoid use.
Materials and Methods
Patients
Eligible subjects were consecutive children with congenital heart disease who had undergone open-heart surgery in the Erasmus MC during a 2-year period. Children were not eligible for the study if they had endocrine or chromosomal abnormalities or had received radiation or chemotherapy within the previous 6 months. The Erasmus MC Medical Ethics Review Board approved the study (196.429/2000/222), and written informed consent was obtained from the parents or legal representatives of each child and of all children older than 12 years.
Clinical Parameters
Anthropometric measurements were taken the day before cardiac surgery. The children were fasted before and during surgery and received glucose intravenously (4–6 mg/kg/min) after surgery according to protocol. Enteral nutrition was initiated on the first postoperative day if clinically possible.
Severity of illness was assessed by Risk Adjustment for Congenital Heart Surgery (RACHS) [10], the pediatric risk of mortality (PRISM) score [19], the pediatric logistic organ dysfunction (PELOD) score [14], and levels of established biomarkers such as interleukin-6 (IL-6), IL-10, and arterial lactate.
Congestive heart failure was defined by the criteria of Van der Kuip et al. [27] adjusted for age. The presence of cyanotic heart disease, the duration of CPB, and the aorta cross-clamp time were recorded.
During cardiac surgery, most of the children received mild hypothermia (median, 31°C), and one child (age, 16.6 years) received deep hypothermia (22.5°C). All the children received standardized analgesia during and after surgery. Glucocorticoids were administered at the discretion of the attending anesthetist. The decision to administer glucocorticoids was made before the start of surgery and independently of the operative course. Standardized protocols were used for administration of inotropes and weaning from the ventilator. The weighted inotropic (WI) score based on maximum inotropic support during surgery and intensive care unit (ICU) stay was calculated [33]. Duration of mechanical ventilation, presence of wound infections, length of ICU and hospital stays, and survival were recorded.
Collection of Blood and Assays
Arterial blood samples were obtained at the start of surgery after induction of anesthesia, at the end of surgery after sternal closure, and then 12 and 24 h afterward. All laboratory parameters except cytokines were determined immediately. Serum and plasma were stored at −80°C until assayed.
Glucose and lactate were determined on an ABL 725 blood gas analyser (Radiometer; Copenhagen, Denmark) in a certified clinical chemistry laboratory (ISO 17025 and 9001). Hypoglycemia was defined as a blood glucose level of 2.2 mmol/l (≤40 mg/dl) or lower and hyperglycemia as a blood glucose level of 8.3 mmol/l (>150 mg/dl) or higher [30]. The normal value for lactate was less than 2.0 mmol/l.
Serum insulin concentrations were determined with an immunoradiometric assay on an Immulite 2000 (Diagnostic Product Corporation, Los Angeles, CA, USA) with a minimum detection level of 35 pmol/l [6]. In our laboratory, the maximum fasting reference value for insulin is 180 pmol/l. The insulin/glucose ratio was calculated to assess insulin sensitivity. To date, no strict reference values exist for the (non)-fasting glucose-to-insulin ratio. In our study, the maximum reference value for the insulin–glucose ratio was defined as 18 pmol/mmol. We derived this value from current literature data, taking into account the differences between insulin assays and units of analysis [5, 28, 32].
Serum cortisol concentrations were determined using an Immulite 2000 competitive luminescence immunoassay (DPC) with detection limits of 3–1,380 nmol/l. The normal level of cortisol during stress was defined as a cortisol level higher than 496 nmol/l [17]. Plasma adrenocorticotrope hormone (ACTH) concentrations were determined by an immunoradiometric assay (Bio International, Gif sur Yvette, France). The within- and between-assay variation coefficients for the assays of cortisol and ACTH were less than 7%.
Plasma cytokine levels were analyzed with an enzyme-linked immunosorbent assay (Sanquin, Amsterdam, The Netherlands). The detection limit of IL-6 (lowest positive standard) was 10 pg/ml, and that of IL-10 was 25 pg/ml.
Statistical Analysis
Data were analyzed with SPSS 16.0 (SPSS inc., Chicago, IL, USA). The results are expressed as median (interquartile range) unless specified otherwise. The Mann–Whitney U test, the chi-square test, and Fisher’s exact test were used for group comparison. Univariate analysis of variance (ANOVA) was used to assess relationships between glucose, steroid use, and disease severity as expressed by WI score and CPB time. Data were log-transformed when necessary. Two-tailed p values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
The study group consisted of 49 children (24 boys) ages 2 months to 18 years. The children had surgery for left–right shunt patch closure of ventricular septal defect (n = 13, including 6 children with combined closure of atrial septal defect and 1 child with additional repair of the tricuspid valve), closure of atrial septal defect (n = 7), patch closure of the aortopulmonary window together with reimplantation of anomalous left coronary artery from the pulmonary artery (n = 1); corrective surgery for tetralogy of Fallot (n = 9), univentricular heart (partial cavopulmonary connection, n = 4; total cavopulmonary connection, n = 2), left ventricular outflow tract obstruction (enucleation, n = 4; pulmonary autograft, n = 2; allograft aortic root replacement, n = 2), right ventricular outflow tract obstruction (infundibulectomy, n = 2; pulmonary allograft, n = 1), and mitral valve insufficiency (mitral valve annuloplasty, n = 2).
Clinical Parameters
All the children underwent elective cardiac surgery upon CPB support, and 45 of the children underwent cardioplegic arrest. All survived.
At the end of surgery, 30 of the children were receiving inotropic support, with 16 receiving dopamine, 6 receiving dobutamine, 7 receiving both dopamine and dobutamine, and 1 receiving noradrenalin.
During cardiac surgery, 32 (65%) of the children received one bolus of glucocorticoids. The bolus was received by 12 children after induction of anesthesia before surgical incision, 8 children at the start of heparinization before CPB, and 12 children at aortic cross-clamping. All except two of the children received methylprednisolone (30 mg/kg), with one child receiving dexamethasone (1 mg/kg) and one child receiving hydrocortisone (2 mg/kg).
For the purpose of this study, we created two groups: those treated with glucocorticoids (n = 32) and those treated without glucocorticoids (n =17). No wound infections occurred. None of the patients received insulin during surgery or ICU stay. The clinical parameters are depicted in Table 1.
Time Courses of Laboratory Parameters
Table 2 shows the laboratory results at the start of surgery, at the end of surgery, and at 12 and 24 h after surgery for the group as a whole.
Glucose
Table 2 shows the blood glucose levels from the start of surgery up to 24 h after surgery. Hypoglycemia (≤2.2 mmol/l [≤40 mg/dl]) did not occur. In general, blood glucose levels were highest at the end of surgery.
At start of surgery, hyperglycemia (>8.3 mmol/l [>150 mg/dl]) was present in one patient. At the end of surgery, hyperglycemia was present in 52% (25/48) of the children, decreasing to 11% (5/47) after 12 h and to 6% (3/47) after 24 h. Thus, almost all the children were normoglycemic after 24 h. Hyperglycemia was not associated with ventilation days nor with the length of the ICU or hospital stays.
Plasma Insulin and Insulin–Glucose Ratios
Table 2 shows the endogenous plasma insulin levels and the insulin–glucose ratios from the start of surgery to 24 h after surgery. At the start of surgery, the plasma levels of insulin in all the children were below the maximum fasting reference level. In all but one of the children (98%), the insulin–glucose ratios were below the maximum reference value.
At the end of surgery, the plasma levels of insulin in 6% (3/48) of the children were above the maximum reference level. The insulin–glucose ratio was increased more than 18 pmol/mmol in 9% (4/47) of the children. They had blood glucose levels varying between 7.4 and 10.8 mmol/l. Of the remaining children, with an insulin–glucose ratio of 18 pmol/mmol or less, 53% (23/43) had hyperglycemia.
At 12 h after surgery, none of the children had plasma insulin levels or an insulin–glucose ratio above the maximum reference value. The insulin levels and insulin–glucose ratios were highest 24 h after surgery. The plasma insulin levels in 9% (4/46) of the children were above the maximum reference level. The insulin–glucose ratio was increased more than 18 pmol/mmol in 20% (9/46) of the children, but only three of them were hyperglycemic. In the remaining children, with an insulin–glucose ratio less than 18 pmol/mmol, hyperglycemia did not occur.
Influence of Glucocorticoids
During surgery, 65% (32/49) of the children were treated with glucocorticoids. The clinical parameters before surgery did not differ between the children with and those without glucocorticoid treatment except for the prevalence of cyanotic heart disease and the WI score, both of which were significantly higher in the children with steroid treatment (Table 1). The laboratory results at the various time points are shown in Table 2 and Fig. 1.
Time course of blood levels of glucose (a), insulin (b), insulin–glucose ratios (c), and cortisol (d) at the start of surgery, at the end of surgery, and 12 and 24 h after surgery for patients treated with and those treated without glucocorticoids. Box–whisker plots: the boxes indicate the 25th to the 75th percentiles with the median, and the attached whiskers indicate the complete range with the exclusion of outliers (open circle) and extremes (*)
The blood glucose levels at the start of surgery, before glucocorticoid treatment, did not differ between the groups (Fig. 1a). At the end of surgery, the blood glucose levels in the children treated with glucocorticoids were significantly higher than in those without glucocorticoid treatment. Hyperglycemia occurred significantly more often in the group that had glucocorticoid treatment (p = 0.001).
The effect of glucocorticoid treatment on the blood glucose levels at the end of surgery was independent of other parameters such as glucose intake, presence of cyanotic heart disease, WI score, and CPB time. At 12 and 24 h after surgery, the median blood glucose levels did not differ between the groups. The insulin levels and insulin–glucose ratios did not differ between the groups at any time point (Fig. 1b, c).
The maximum peak cortisol levels were found at the end of surgery, with significantly higher cortisol levels and cortisol–ACTH ratios in the children treated with glucocoricoids (Fig. 1d). At 12 and 24 h after surgery, the cortisol levels and cortisol–ACTH ratios of the children with glucocorticoid treatment had spontaneously decreased to the levels in the children without glucocorticoid treatment. In both groups, however, the cortisol levels still were higher than the levels at the start of surgery. The IL-6 level 12 and 24 h after surgery was significantly lower in the children treated with glucocorticoids. The IL-10 level at the end of surgery and 12 h after surgery was significantly higher in the children treated with glucocorticoids. There were no other differences in laboratory parameters between the two groups.
Discussion
Our study showed that treatment with glucocorticoids during surgery was the main factor associated with the occurrence of hyperglycemia at the end of CPB surgery for congenital heart defects. Hyperglycemia frequently occurred with the highest blood glucose levels at the end of surgery and disappeared spontaneously (without insulin therapy) within 12–24 h in the majority of the children without significant postoperative morbidity.
The occurrence of hyperglycemia was not associated with increased morbidity, as shown by duration of ventilation, ICU stay, or hospital stay. Moreover, the overall morbidity in our population was low. The median duration of mechanical ventilation was 9 h, whereas the ICU say was 2 days and the hospital stay was 7 days. Renal dialysis and extracorporeal life support did not occur, and all the patients survived.
Hyperglycemia in critically ill children is caused by multiple proposed mechanisms including counterregulatory hormone-mediated upregulation of gluconeogenesis and glycogenolysis and downregulation of glucose transporters with decreased peripheral use of glucose by tissues such as skeletal muscle and liver [29].
In the current study, we evaluated how many hyperglycemic patients showed signs of insulin resistance because this is described as the main factor causing hyperglycemia in critically ill adults [23, 26, 36]. In only four children (9%) was an increased insulin–glucose ratio (>18 pmol/mmol) seen at the end of surgery. The remaining hyperglycemic children showed a normal or (relatively) decreased insulin–glucose ratio. The plasma insulin levels increased 24 h after surgery, which might have been due to the fact that most patients were detubated and already receiving enteral nutrition. The increase in insulin levels can be interpreted as a recovery response to the administered enteral feeding.
The decreased insulin response after surgery might be due to the fact that critically ill children seem to be more vulnerable than adults to beta-cell dysfunction. Preissig and Rigby [20] hypothesized that beta cells, known to be exquisitely sensitive to rapid physiologic changes, may become dysfunctional if these changes occur acutely above a certain threshold. These changes may be induced by multiple factors such as hypothermia, vasopressors, elevations of proinflammatory cytokines, and use of glucocorticoids [1, 9, 12, 20].
In the study by Preissig and Rigby [20], the vasopressor score of critically ill children with respiratory and cardiovascular failure was inversely correlated with the C-peptide level, indicating beta-cell dysfunction due to the suppressing effect of exogeneous catecholamines. In our study, the use of vasopressors was low, with only one patient receiving noradrenalin. This may explain the relatively normal plasma insulin levels at the end of surgery.
Another explanation for the less pronounced hypoinsulinemic response in our study might be the mild effect of cardiac surgery on the inflammatory response, as shown by the low levels of Il-6 and the mildly increased IL-10 at the end of surgery in the patients without glucocorticoid treatment. Perioperative administration of glucocorticoids was associated with decreased IL-6 and increased IL-10 levels after CPB. This accords with adult studies showing that glucocorticoids may decrease the inflammatory response during the CPB procedure [3]. However, for pediatric patients with congenital heart disease undergoing CPB surgery, the clinical benefit of this suppressed cytokine response remains unclear.
Debate exists about the positive effects of steroid use during CPB in pediatric patients and whether the potential positive effects of corticosteroid treatment during CPB surgery outweigh the potential adverse effects, such as hyperglycemia [4, 22]. High blood glucose levels at the end of CPB surgery for congenital heart defects also were found in previous studies [1, 7, 21, 24]. We found a spontaneous normalization of blood glucose levels within 24 h postoperatively, which is in line with the finding of one other study [24]. However, a few other studies show a more gradual decrease in blood glucose levels over 3 days [18, 35]. This could be related to our relatively low postoperative morbidity compared with other studies and comparable preoperative illness severity as expressed by RACHS.
Other authors [21] have reported an increase in ICU stay (median, 3–6 days), mechanical ventilation (4.4 days), dialysis (1.1–4%), extracorporeal life support (3–8%), and mortality (4–11%). Vlasselaers et al. [31] reported their results from a prospective randomized controlled trial of treatment for critically ill children (75% were patients after cardiac surgery for congenital heart defects). Intensive insulin therapy for hyperglycemia improved morbidity and reduced mortality, but the harm of insulin-induced hypoglycemic events is debated [11]. It is important to realize that not only hyperglycemia but also hypoglycemia is associated with an adverse outcome [7, 25, 34, 35].
In general, important differences exist between centers in terms of morbidity and mortality after pediatric cardiac surgery. In our study, none of the patients were treated with insulin for hyperglycemia, and overall morbidity was low, so the standard use intensive insulin therapy is not needed for tight glycemic control.
A limitation of this study was that glucocorticoids were administered at the discretion of the attending anesthetist. Although treatment was not randomized, glucocorticoids were administered before aortic clamping and thus were independent of the operative course. Moreover, there were no differences in age, clinical course, or duration of CPB time between the patients with and those without glucocorticoid treatment. Furthermore, although the patients with cyanotic heart disease were more likely to receive glucocorticoids and although the median WI score was higher for the glucocorticoid-treated patients, univariate analysis of variance showed that preoperatively administered glucocorticoids were independently associated with increased blood glucose levels at the end of surgery.
In summary, our study showed that the development of hyperglycemia at the end of cardiac surgery for congenital heart disease was associated with glucocorticoid administration during surgery. Postoperative hyperglycemia was frequent, but in almost all cases (94%), blood glucose levels spontaneously normalized within 24 h without the use of insulin administration and without significant morbidity or mortality. The standard use of intensive insulin therapy for tight glycemic control is not needed in this patient group. In contrast to our hypothesis, we conclude that because postoperative morbidity was low in the study group, the presumed positive effects of glucocorticoids seemed to outweigh the adverse effects of iatrogenic hyperglycemia.
Future research should focus on the value of glucocorticoid therapy during pediatric cardiac surgery, weighing both the pros and cons of either hyperglycemia or glucocorticoid therapy.
References
Benzing G III, Francis PD, Kaplan S, Helmsworth JA, Sperling MA (1983) Glucose and insulin changes in infants and children undergoing hypothermic open heart surgery. Am J Cardiol 52:133–136
Bouwmeester NJ, Anand KJ, van Dijk M, Hop WC, Boomsma F, Tibboel D (2001) Hormonal and metabolic stress responses after major surgery in children aged 0–3 years: a double-blind, randomized trial comparing the effects of continuous versus intermittent morphine. Br J Anaesth 87:390–399
Celik JB, Gormus N, Okesli S, Gormus ZI, Solak H (2004) Methylprednisolone prevents inflammatory reaction occurring during cardiopulmonary bypass: effects on TNF-alpha, IL-6, IL-8, IL-10. Perfusion 19:185–191
Chaney MA (2002) Corticosteroids and cardiopulmonary bypass: a review of clinical investigations. Chest 121:921–931
Conwell LS, Trost SG, Brown WJ, Batch JA (2004) Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care 27:314–319
den Brinker M, Joosten KF, Liem O, de Jong FH, Hop WC, Hazelzet JA, van Dijk M, Hokken-Koelega AC (2005) Adrenal insufficiency in meningococcal sepsis: bioavailable cortisol levels and impact of interleukin-6 levels and intubation with etomidate on adrenal function and mortality. J Clin Endocrinol Metab 90:5110–5117
Falcao G, Ulate K, Kouzekanani K, Bielefeld MR, Morales JM, Rotta AT (2008) Impact of postoperative hyperglycemia following surgical repair of congenital cardiac defects. Pediatr Cardiol 29:628–636
Furnary AP (2009) Clinical benefits of tight glycaemic control: focus on the perioperative setting. Best Pract Res Clin Anaesthesiol 23:411–420
Gesina E, Tronche F, Herrera P, Duchene B, Tales W, Czernichow P, Breant B (2004) Dissecting the role of glucocorticoids on pancreas development. Diabetes 53:2322–2329
Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI (2002) Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 123:110–118
Joosten K, Verbruggen SC, Verhoeven JJ (2009) Glycaemic control in paediatric critical care. Lancet 373:1423–1424 (author reply 1424)
Lambillotte C, Gilon P, Henquin JC (1997) Direct glucocorticoid inhibition of insulin secretion: an in vitro study of dexamethasone effects in mouse islets. J Clin Invest 99:414–423
Lazar HL, McDonnell M, Chipkin SR, Furnary AP, Engelman RM, Sadhu AR, Bridges CR, Haan CK, Svedjeholm R, Taegtmeyer H, Shemin RJ (2009) The Society of Thoracic Surgeons practice guideline series: blood glucose management during adult cardiac surgery. Ann Thorac Surg 87:663–669
Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, Gottesman R, Joffe A, Pfenninger J, Hubert P, Lacroix J, Leclerc F (2003) Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet 362:192–197
Marik PE, Raghavan M (2004) Stress hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med 30:748–756
Mizock BA (2001) Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab 15:533–551
Parker MM, Hazelzet JA, Carcillo JA (2004) Pediatric considerations. Crit Care Med 32:S591–S594
Polito A, Thiagarajan RR, Laussen PC, Gauvreau K, Agus MS, Scheurer MA, Pigula FA, Costello JM (2008) Association between intraoperative and early postoperative glucose levels and adverse outcomes after complex congenital heart surgery. Circulation 118:2235–2242
Pollack MM, Ruttimann UE, Getson PR (1988) Pediatric risk of mortality (PRISM) score. Crit Care Med 16:1110–1116
Preissig CM, Rigby MR (2009) Hyperglycaemia results from beta-cell dysfunction in critically ill children with respiratory and cardiovascular failure: a prospective observational study. Crit Care 13:R27
Preissig CM, Rigby MR, Maher KO (2009) Glycemic control for postoperative pediatric cardiac patients. Pediatr Cardiol 30:1098–1104
Robertson-Malt S, Afrane B, El Barbary M (2007) Prophylactic steroids for pediatric open heart surgery. Cochrane Database Syst Rev CD005550
Robinson LE, van Soeren MH (2004) Insulin resistance and hyperglycemia in critical illness: role of insulin in glycemic control. AACN Clin Issues 15:45–62
Rossano JW, Taylor MD, Smith EO, Fraser CD Jr, McKenzie ED, Price JF, Dickerson HA, Nelson DP, Mott AR (2008) Glycemic profile in infants who have undergone the arterial switch operation: hyperglycemia is not associated with adverse events. J Thorac Cardiovasc Surg 135:739–745
Srinivasan G, Jain R, Pildes RS, Kannan CR (1986) Glucose homeostasis during anesthesia and surgery in infants. J Pediatr Surg 21:718–721
Van den Berghe G (2004) How does blood glucose control with insulin save lives in intensive care? J Clin Invest 114:1187–1195
van der Kuip M, Hoos MB, Forget PP, Westerterp KR, Gemke RJ, de Meer K (2003) Energy expenditure in infants with congenital heart disease, including a meta-analysis. Acta Paediatr 92:921–927
van Waardenburg DA, Jansen TC, Vos GD, Buurman WA (2006) Hyperglycemia in children with meningococcal sepsis and septic shock: the relation between plasma levels of insulin and inflammatory mediators. J Clin Endocrinol Metab 91:3916–3921
Vanhorebeek I, Langouche L, Van den Berghe G (2005) Glycemic and nonglycemic effects of insulin: how do they contribute to a better outcome of critical illness? Curr Opin Crit Care 11:304–311
Verbruggen SC, Joosten KF, Castillo L, van Goudoever JB (2007) Insulin therapy in the pediatric intensive care unit. Clin Nutr 26:677–690
Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G (2009) Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet 373:547–556
Vuguin P, Saenger P, Dimartino-Nardi J (2001) Fasting glucose insulin ratio: a useful measure of insulin resistance in girls with premature adrenarche. J Clin Endocrinol Metab 86:4618–4621
Wernovsky G, Wypij D, Jonas RA, Mayer JE Jr, Hanley FL, Hickey PR, Walsh AZ, Chang AC, Castaneda AR, Newburger JW et al (1995) Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants: a comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 92:2226–2235
Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM (2006) Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics 118:173–179
Yates AR, Dyke PC II, Taeed R, Hoffman TM, Hayes J, Feltes TF, Cua CL (2006) Hyperglycemia is a marker for poor outcome in the postoperative pediatric cardiac patient. Pediatr Crit Care Med 7:351–355
Zauner A, Nimmerrichter P, Anderwald C, Bischof M, Schiefermeier M, Ratheiser K, Schneeweiss B, Zauner C (2007) Severity of insulin resistance in critically ill medical patients. Metabolism 56:1–5
Acknowledgments
The authors acknowledge research nurse M. Maliepaard for her assistance in data collection and K. Hagoort for his careful editing. They also are grateful to Dr. D. Tibboel for critically reviewing the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Verhoeven, J.J., Hokken-Koelega, A.C.S., den Brinker, M. et al. Disturbance of Glucose Homeostasis After Pediatric Cardiac Surgery. Pediatr Cardiol 32, 131–138 (2011). https://doi.org/10.1007/s00246-010-9829-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-010-9829-z