Abstract
Microplastics are highly persistent particles that deliberately contaminate our ecosystem. These small-sized particles can pass through filtering systems into the water bodies, affecting various forms of aquatic and terrestrial life. However, little is known about their fragmentation process within the organism’s body. In previous studies, commercially available microplastics were used that are rarely found in the environment naturally, hence they cannot mimic the effects on our surroundings. Therefore, using the zebrafish, Danio rerio we have evaluated the process of bio-fragmentation of ingested pristine polyethene microplastics which are widely used in our daily life. We have also examined their faecal pellets through Field Emission Scanning Electron Microscopy (FE-SEM) and Fourier transform infrared spectroscopy (FTIR). Our results show that zebrafish can potentially bio-fragment the pristine microplastic particles into nano-plastic within a short period of 24 h. Additionally, zebrafish cannot recognize the pristine microplastic particles and can ingest them as food. No mortality occurred during the experiment. Thus, we have identified a natural pathway of microplastic bio-fragmentation, introducing an emerging role of zebrafish in biogeochemical cycling and the fate of plastics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Microplastics are a growing global issue. When plastic gets discharged into the environment, it degrades, causing severe problems (Zalasiewicz et al. 2016). Because of their tiny size, a significant number will pass through filtering systems and into water bodies, affecting aquatic life (Wang et al. 2018; Miraj et al. 2019). In the year 2020, The COVID-19 pandemic increased the production and consumption of plastics in the form of facemasks, face shields, and personal protective equipment (PPE) kits, that got degraded, exacerbating the burden of microplastics on the environment (Dharmaraj et al. 2021).
Fish is an essential component of the food chain, and fishmeal is a potential pathway for microplastic contamination into the aquatic environment (Thiele et al. 2021). The processes of bioaccumulation and biotransformation of microplastics in fishes is well documented (Cedervall et al. 2012; Lu et al. 2016; Limonta et al. 2021). However, no research has been reported on the biological fragmentation of microplastics in fishes which are ingested as food. The biological fragmentation of polyethene particles in soil is often associated with microbial degradation (Paço et al. 2017; Dey et al. 2020; Khan et al. 2022), which proceeds slowly due to the limited availability of oxygen, light, and microorganisms capable of the polymer degradation (Rogers et al. 2020). Similarly, the bio-fragmentation of microplastics have also been reported for some marine invertebrates such as Antarctic krill (Euphausia superba), Amphipod (Hyalella azteca) (Dawson et al. 2018; Borges et al. 2022).
In laboratory, researchers have acquired microplastic particles from scientific vendors of precise size and shape (Espinosa et al. 2018; Yang et al. 2019; Malafaia et al. 2020). These microplastic particles have uniform sizes and smooth textures which might pass and unaffected through, or might sufficiently too small to translocate in the organism body (Dawson et al. 2018), whereas the microplastics found in environment are degraded plastic polymers having irregular in sizes and rough surfaces which may retain in organism body for longer period and can mimic the natural effects of microplastics in our delicate ecosystem. Therefore, it is necessary to understand the impact of pristine microplastic particles (PEMPs) that are present in the environment.
In the present study, we have focussed on the biological fragmentation of pristine polyethene microplastics (PEMPs) eaten by zebrafish and have performed chemical structure analysis of PEMPs before ingestion and of the egested faecal pellets of zebrafish containing PEMPs through Fourier Transform Infrared Spectroscopy (FTIR) and morphological studies by Field Emission Scanning Electron microscopy (FE-SEM). The primary objective of this study is to identify the biological fragmentation process of PEMPs eaten by zebrafish and to demonstrate that zebrafish can potentially ingest pristine polyethene microplastic particles (PEMPs) and egest them by converting them into fragmented nano-plastics. In the present research, the high concentration of PEMPs (20 mg/L) was selected for zebrafish exposure to inculcate the severe response during the experiment. The tested concentrations are equivalent to 9.4 × 107 particles/m3, quantified according to Leusch & Ziajahromi (2021) protocol. In surface water the MPs concentration can range from 1 × 10−2 to 108 particles/m3 (Koelmans et al. 2019). Therefore, the exposed PEMPs concentration was environmentally relevant concentration in the present plastic pollution scenario.
Materials and Methods
Microplastic Characterization and Dispersion
Pristine microplastic particles were collected from polyethene-making industry in Bhopal (M.P). Microplastic particles were confirmed to be polyethene by Fourier Transform Infrared Spectroscopy, FTIR (3000 Hyperion Microscope with Vertex 80 FTIR System). The size and morphology of PEMPs were determined through Field Emission Scanning Electron microscopy, FE-SEM (Zeiss ULTRA Plus). For experimentation, pristine microplastic particles were treated with Tween 80, a surfactant used to disperse microplastic particles in an aqueous solution. PEMPs were soaked in 0.01% of Tween 80 for 24 h or until equal dispersion, (Lu et al. 2016). Then, PEMPs were filtered with Whatman Grade 1 filter paper. Further, microplastic particles were dispersed in sterile aerated water.
Zebrafish Housing and Acclimatization
8–10 months old zebrafish adult of both sexes (0.6 g ± 0.4 g; 3.5 cm ± 2 cm total length-mean ± SD-proportion 1:1) were purchased from commercial dealer in Bhopal, Madhya Pradesh, India, and were acclimatized for 15 days in laboratory conditions, in dechlorinated water providing photoperiod of 14 h light: 10 h dark. Temperature range of 25 °C–27 °C, pH 7 -7.5 and dissolved oxygen of 2–4 ppm were maintained. Fishes were fed micropellets twice a day, (Westerfield et al. 2000; Kim et al. 2017).
Experimental Design
After acclimatization, 12 healthy zebrafish were transferred into (n = 6, zebrafishes) in control group and (n = 6, zebrafishes) in experimental group. Both groups were kept in the small tanks (20 cm × 15 cm × 15 cm) filled with 2 L of water. The experimental group was exposed to water containing pristine polyethene microplastic particles (PEMPs). The fishes of the control group were maintained in PEMP-free aerated water. Upon transfer to the experimentation tank, zebrafish started consuming microplastics immediately present in the aquarium water. Control fishes were fed with commercially available food pellets (betta diet). The egested particles also contained organic matter as they were found into the bottom of the tank. After 24 h, the zebrafishes were removed from the tank and the faecal pellets were collected from both experimental and control tanks and observed under a light microscope with 40X resolution. To confirm microplastic fragmentation, the faecal pellets were washed with distilled water and air dried for 48 h. The dried pellets were observed under light microscope, and no faecal matter was observed with the particles.
Sample Analysis
The ingested and egested PEMPs particles were examined through Fourier Transform Infrared Spectroscopy (FTIR) and further analysed using Field Emission Scanning Electron Microscopy (FESEM), (Wang et al. 2017; Truchet et al. 2021). Approximately 100 ± 20 PEMPs particles were observed through FESEM from the control (PEMPs before ingestion) and experiment (egested PEMPs). SEM photomicrographs of PEMPs particles were examined with Image J software for particle size measurements. Also, Leusch and Ziajahromi, (2021) method was adopted for PEMPs particle counting.
Results
In the present study, we have observed the process of biological fragmentation of ingested microplastics by zebrafish, Danio rerio. Polymer identification and chemical structure alterations by FTIR spectroscopy. In our experiment, FTIR spectroscopy confirmed the microplastic particles are polyethene particles. FTIR spectra of PEMPs before ingestion (control) are illustrated in Fig. 1A depicting peaks that belonged to polyethene at 3435.26 cm−1 indicated (O–H stretching), 2919.78 cm−1 & 2851 cm−1 indicated (C–H bond stretching). The peaks obtained at 1627.51 cm−1 (C=C stretching alkene), 1542.72 cm−1 (CH2 bending vibration), 1485.56 cm−1 and 1384.13 cm−1 (CH3 methyl bending deformation) & the peak at 719.44 cm−1 were determined to be due to (CH2 rock vibration) mode.
After 24 h of ingestion of the microplastics, zebrafish egested faecal pellets containing fragmented microplastics which were examined through Fourier Transform Infrared Spectroscopy (FTIR) as illustrated in Fig. 1B depicts the generation of new peaks at 1030.77 cm−1 (S–O stretching) and 873.88 cm−1 (C-H bending). The reduction in peaks in comparison to control PEMPs was also been observed at 1467 cm−1 (C–H bending alkane), 718 cm−1 (C=C stretching alkene), 1463.10 cm−1 (C–H bending alkane), 2921.35 cm−1 & 2851.52 cm−1 (C–H bond stretching). The overlapping peaks 3447.22 cm−1 and 3421.46 cm−1 with higher intensity were observed corresponding to (O–H stretching) alcohols representing the chemical structure alteration on the surface of the egested PEMPs. The absorption peaks generated in the fingerprint region (1500 cm−1–500 cm−1) of control PEMPs (microplastic before ingestion) are different from the egested PEMPs representing the change in the chemical structure of PEMPs. Thus, the FTIR spectroscopy results suggested that the chemical structure of PEMPs gets biologically altered after being digested by zebrafish.
We have identified the morphological alterations in the PEMPs by Field Emission Scanning Electron microscopy (FE-SEM). We have examined PEMP particles before ingestion (control) and egested PEMPs in faecal pellets (experimental) as illustrated in Fig. 2A and B. The surface of egested PEMPs becomes rough, and cracks had appeared. Thus, the reduced size and significant alterations in their morphology suggest that the egested PEMPs get breakdown into nano-plastic particles. Approximately 100 ± 20 PEMPs particles were observed through SEM from the control (PEMPs before ingestion) and experiment (egested PEMPs). SEM photomicrographs of PEMPs particles were examined with ImageJ software for particle size measurements. The observed size range of PEMPs before ingestion is between 10 μm and 100 μm with mean diameter of 76.74 μm (± 14.07 standard deviation, S.D), whereas the size range of egested PEMPs is between 100 nm and 10 μm with mean diameter of 5.92 μm (± 4.96 standard deviation, S.D) which shows approximately 70% size reduction in PEMPs particles after digested by the zebrafishes.
Discussion
The zebrafish do not have a true stomach. Without a stomach, digestion and absorption must begin as early as possible in the limited length of the zebrafish digestive tract. Its digestive system is however segmented into the mouth, oesophagus, three gut segments (anterior, middle, and posterior), and anus. The zebrafish oesophagus is connected with the anterior gut segment, where nutrient absorption predominantly occurs due to the high presence of digestive enzymes. Under normal physiological conditions, the pH of the intestinal lumen of zebrafish is alkaline (> 7.5) (Nalbant et al. 2004; Flores et al. 2020). Ingested food is temporarily stored in the rostral intestinal bulb that bulges like an elastic sac, where food starts to be broken down in the absence of a stomach, and the entire length of the intestine may serve to degrade food (Kapoor et al. 1975; Wallace et al. 2005; Nadal et al. 2020). Our results are inline with these findings, it might be possible that PEMP particles get fragmented in the zebrafish intestine due to the presence of different digestive enzymes and microbiomes in the digestive system.
Our results clearly demonstrate that zebrafish can significantly alter the structural integrity and cause reduction in the size of microplastics engulfed in feeding by a process called bio-fragmentation. We have identified that zebrafish readily ingested and egested the microplastics within 24 h. In a previous study, Micusik et al. (2021) reported degradation of LDPE which was accidentally found in the stomach of catfish in the Bodrog River, Slovakia. On the contrary, we have performed a series of a controlled experiments which demonstrate the gradual feed intake of pristine polyethene microplastics particles by the zebrafish which gets bio-fragmented or bio-degraded, followed by their egestion as smaller fragments of microplastics.
During the experiment, we also observed that zebrafish got confused with the microplastic particles as food and consumed all of them, as reported earlier by Ory et al. (2018) & Lusher et al. (2020). After 24 h of ingestion, we have observed the significant colour differences in the faecal pellets of zebrafishes of the control and experimental fishes. The faecal pellets of both control and experimental fishes were analysed under a light microscope (40 × resolution) and found to be microplastic particles in the faecal pellets of PEMPs exposed zebrafish, illustrated in Fig. 3. There were no mortalities or any kind of adverse effects on the body condition of fishes during the feeding with PEMPs.
Fourier Transform Infrared Spectroscopy (FTIR) has been widely used to identify the polymer and changes in the chemical structure of the polymer (Chalmers and Dent. 2006; Veerasingam et al. 2020). The observed FTIR spectra of PEMPs particles before ingestion (control) showed the incorporated carbonyl groups, carboxylic acids, hydroxyl groups and alkene double bonds confirming the microplastics is low density polyethylene (LDPE). In our observations, FTIR spectra have exhibited the generation of peak at 3435.26 cm−1 representing O–H bond stretching, while peaks at 2919.78 cm−1 and 2851 cm−1 representing CH bond stretching. The peak that was also found at 1627.51 cm−1, which representing C=C stretching alkene bonds, and the peak that was found at 1542.72 cm−1, which showed CH2 bending vibration. In addition, we also identified the peaks at 1485.56 cm−1 and 1384.13 cm−1 exhibited CH3 methyl bending deformation. These peaks, along with the peak that was observed at 719.44 cm−1, were a result of the rock vibration mode. Our investigations are in agreement with those of D’Souza et al. (2021), who also found similar peaks in LDPE.
In our study, FTIR spectroscopy also confirmed the level of structural changes in egested PEMPs by the zebrafish. During the process of microbial biodegradation microorganisms secrete various extracellular enzymes, that facilitate a number of chemical reactions that may lead to alteration in chemical conversions, including oxidation, reduction, esterification, hydrolysis, and inner molecular conversion. These chemical reactions reflected the change in the PE carbon backbone by the formation of various new functional groups and dissolution of older groups, indicating biodegradation process which has also been demonstrated by Muhonja et al. (2018) and Khan et al. (2022). Similarly, the FTIR spectra of egested PEMPs showed the variations in older peaks and the formation of new peaks confirming the biological oxidation or degradation of pristine microplastic particles by the zebrafishes.
Field Emission Scanning Electron Microscopy (FE-SEM) allows the determination of biodegradation of polymers by investigating the morphological properties. Using this FESEM, we have found that fish egested PEMPs developed cracks and holes at the interface with approximately 70% reduction in the size of PEMPs from 100 μm to 100 nm mean diameter of 5.92 μm (± 4.96 standard deviation, S.D) as illustrated in Fig. 4. Surface erosion, cracks and holes have been observed in the photomicrographs of egested PEMPs particles. These characteristics of egested PEMPs were similar to the previous studies of polyethylene biodegradation. This confirms the degradation of the microplastics by the zebrafish during feeding and egestion process. This is in conformation of a report by Wang et al. (2017), who also found development of cracks in the PVC particles which were obtained from ocean trawl and fish guts using Field Emission Scanning Electron Microscopy.
Conclusion
The findings of the present study indicate that zebrafish can potentially bio-fragment the pristine microplastic particles, consumed during the process of feeding, within a short period of 24 h. Physical and chemical alterations on the surface of the polymers were the results of bio-fragmentation of microplastic particles during the process of feeding, ingestion, digestion and egestion. The present work aspires to constitute a stepping stone towards the natural process of biological mitigation of the microplastics. Thus, we have identified a new natural pathway of microplastic bio-fragmentation, introducing an emerging role of zebrafish in biogeochemical cycling and the fate of plastics. Further investigations are needed to understand the role of metabolic enzymes of zebrafish, which highlight the efficient bio-fragmentation of pristine polyethene microplastics at cellular and molecular levels.
Abbreviations
- PEMPs:
-
Pristine polythene microplastics (PEMPs)
- mg:
-
Milligram
- FE-SEM:
-
Field Emission Scanning Electron microscopy
- FTIR:
-
Fourier Transform Infrared Spectroscopy
- COVID-19:
-
Coronavirus disease
- PPE:
-
Personal Protective Equipment
- LDPE:
-
Low Density Polyethene
- PVC:
-
Polyvinyl chloride
References
Borges BR, Queiroz L, Prado C, Melo E, Moraes B, Ando R, Paiva T, Pompêo M (2022) Expressive Biofragmentation of Polystyrene Microplastics by the Amphipod Hyalella Azteca. SSRN Electron J. https://doi.org/10.2139/ssrn.4206912
Cedervall T, Hansson LA, Lard M, Frohm B, Linse S (2012) Food chain transport of nanoparticles affects behaviour and fat metabolism in fish. PloS One 7:e32254. https://doi.org/10.1371/journal.pone.0032254
Chalmers JM, Dent G (2006) Vibrational spectroscopic methods in pharmaceutical solid-state characterization. Polymorphism in the pharmaceutical industry. Wiley, Weinheim, pp 95–138
Dawson AL, Kawaguchi S, King CK, Townsend KA, King R, Huston WM, Bengtson Nash SM (2018) Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat Commun 9:1001. https://doi.org/10.1038/s41467-018-03465-9
Dey AS, Bose H, Sar MB (2020) Biodegradation of unpretreated low-density polyethylene (LDPE) by Stenotrophomonas sp. and Achromobacter sp., isolated from waste dumpsite and drilling fluid. Front Microbiol 11:603. https://doi.org/10.3389/fmicb.2020.603210
Dharmaraj S, Ashokkumar V, Hariharan S, Manibharathi A, Show PL, Chong CT, Ngamcharussrivichai C (2021) The COVID-19 pandemic face mask waste: a blooming threat to the marine environment. Chemosphere 272:132411. https://doi.org/10.1016/j.chemosphere.2021.129601
D’Souza GC, Sheriff RS, Ullanat V, Shrikrishna A, Joshi AV, Hiremath L, Entoori K (2021) Fungal biodegradation of low-density polyethylene using consortium of Aspergillus species under controlled conditions. Heliyon 7(5):e07008. https://doi.org/10.1016/j.heliyon.2021.e07008
Espinosa C, García Beltrán JM, Esteban MA, Cuesta A (2018) In vitro effects of virgin microplastics on fish head-kidney leucocyte activities. Environ Pollut 235:30–38. https://doi.org/10.1016/J.ENVPOL.2017.12.054
Flores EM, Nguyen AT, Odem MA, Eisenhoffer GT, Krachler AM (2020) The zebrafish as a model for gastrointestinal tract-microbe interactions. Cellular Microbiology. 22(3):e13152. https://doi.org/10.1111/cmi.13152
Kapoor BG, Smit H, Verighina IA (1975) The alimentary canal and digestion in Teleosts. Adv Mar Biol 1975(13):109–211. https://doi.org/10.1016/S0065-2881(08)60281-3
Khan S, Ali SA, Ali AS (2022) Biodegradation of low-density polyethylene (LDPE) by mesophilic fungus Penicillium citrinum isolated from soils of plastic waste dump yard Bhopal India. Environ Technol. https://doi.org/10.1080/09593330.2022.2027025
Kim SH, Sharma C, Khan I, Kang SC (2017) Breeding of zebrafish in the laboratory environment for research development. Bangladesh J Pharmacol 12(4):434–438
Koelmans AA, Mohamed Nor NH, Hermsen E, Kooi M, Mintenig SM, De France J (2019) Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res 155:410–422. https://doi.org/10.1016/j.watres.2019.02.054
Leusch FDL, Ziajahromi S (2021) Converting mg/L to Particles/L: reconciling the occurrence and toxicity literature on microplastics. Environ Sci Technol 55(17):11470–11472. https://doi.org/10.1021/acs.est.1c04093
Limonta G, Mancia A, Abelli L, Fossi MC, Caliani I, Panti C (2021) Effects of microplastics on head kidney gene expression and enzymatic biomarkers in adult zebrafish. Compar Biochem Physiol Part C Toxicol Pharmacol 245:109037. https://doi.org/10.1016/J.CBPC.2021.109037
Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, Ren H (2016) Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol 50(7):4054–4060. https://doi.org/10.1021/acs.est.6b00183
Lusher AL, Welden NA, Sobral P, Cole M (2020) Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Analysis of nanoplastics and microplastics in food, 1st edn. CRC Press, Boca raton, pp 119–148
Malafaia G, de Souza AM, Pereira AC, Gonçalves S, da Costa Araújo AP, Ribeiro RX, Rocha TL (2020) Developmental toxicity in zebrafish exposed to polyethylene microplastics under static and semi-static aquatic systems. Sci Total Environ 700:134867. https://doi.org/10.1016/J.SCITOTENV.2019.134867
Micusik M, Kleinova A, Oros M, Simon P, Dubaj T, Prochazka M, Omastová M (2021) Plastic ingestion by the Wels catfish (Silurus glanis L.): Detailed chemical analysis and degradation state evaluation. Toxicol Rep 8:1869–1876. https://doi.org/10.1016/j.toxrep.2021.11.006
Miraj SS, Parveen N, Zedan HS (2019) Plastic microbeads: small yet mighty concerning. Int J Environ Health Res 31:788–804. https://doi.org/10.1080/09603123.2019.1689233
Muhonja CN, Makonde H, Magoma G, Imbuga M (2018) Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS ONE 13(7):e0198446. https://doi.org/10.1371/journal.pone.0198446
Nadal AL, Ikeda-Ohtsubo W, Sipkema D, Peggs D, McGurk C, Forlenza M, Wiegertjes GF, Brugman S (2020) Feed, microbiota, and gut immunity: using the zebrafish model to understand fish health. Front Immunol 11:114. https://doi.org/10.3389/fimmu.2020.00114
Nalbant P, Boehmer C, Dehmelt L, Wehner F, Werner A (2004) Functional characterization of a Na+-phosphate cotransporter (NaP1-II) from zebrafish and identification of related transcripts. J Physiol 520:79–89. https://doi.org/10.1111/j.1469-7793.1999.00079.x
Ory NC, Gallardo C, Lenz M, Thiel M (2018) Capture, swallowing, and egestion of microplastics by a planktivorous juvenile fish. Environ Pollut 240:566–573. https://doi.org/10.1016/j.envpol.2018.04.093
Paço A, Duarte K, da Costa JP, Santos PSM, Pereira R, Pereira ME, Freitas AC, Duarte AC, Rocha-Santos TAP (2017) Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci Total Environ 586:10–15. https://doi.org/10.1016/J.SCITOTENV.2017.02.017
Rogers KL, Carreres-Calabuig JA, Gorokhova E, Posth NR (2020) Micro-by-micro interactions: how microorganisms influence the fate of marine microplastics. Limnol Oceanography Lett 5:18–36. https://doi.org/10.1002/lol2.10136
Thiele CL, Hudson MD, Russell AE, Saluveer M, Sidaoui-Haddad G (2021) Microplastics in fish and fishmeal: An emerging environmental challenge? Sc Rep 11:1–12
Truchet DM, López ADF, Ardusso MG, Rimondino GN, Buzzi NS, Malanca FE, Spetter CV, Severini MDF (2021) Microplastics in bivalves, water and sediments from a touristic sandy beach of Argentina. Marine Pollut Bull 173:113023. https://doi.org/10.1016/J.MARPOLBUL.2021.113023
Veerasingam S, Ranjani M, Venkatachalapathy R, Bagaev A, Mukhanov V, Litvinyuk D, Vethamony P (2020) Microplastics in different environmental compartments in India: analytical methods, distribution, associated contaminants and research needs. TrAC Trends Anal Chem 133:116071. https://doi.org/10.1016/j.marpolbul.2020.111478
Wallace KN, Akhter S, Smith EM, Lorent K, Pack M (2005) Intestinal growth and differentiation in zebrafish. Mech Dev 2005(122):157–173. https://doi.org/10.1016/j.mod.2004.10.009
Wang ZM, Wagner J, Ghosal S, Bedi G, Wall S (2017) SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts. Sci Total Environ 603–604:616–626. https://doi.org/10.1016/J.SCITOTENV.2017.06.047
Wang J, Zheng L, Li J (2018) A critical review on the sources and instruments of marine microplastics and prospects on the relevant management in China. Waste Manage Res 36:898–911. https://doi.org/10.1177/0734242X18793504
Westerfield M (2000) The zebrafish book: a guide for the laboratory use of zebrafish, 4th edn. University of Oregon Press, Eugene
Yang J, Cang L, Sun Q, Dong G, Ata-Ul-Karim ST, Zhou D (2019) Effects of soil environmental factors and UV aging on Cu2+ adsorption on microplastics. Environ Sci Pollut Res 26:23027–23036. https://doi.org/10.1007/s11356-019-05643-8
Zalasiewicz J, Colin NW, Juliana I (2016) The geological cycle of plastics and their use as a stratigraphic indicator of the Anthropocene. Anthropocene 13:4–17. https://doi.org/10.1016/j.ancene.2016.01.002
Acknowledgements
The authors are thankful to the Secretary and Principal of Saifia College of Science, Bhopal, India, for providing the necessary facilities.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Darakhshan Khan and Dr. Sharique A. Ali. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical Approval
The present experimental work was carried out in the laboratory of the Department of Biotechnology, Saifia Science College Bhopal, India, approved by Institutional Animal Ethics Committee (IAEC) under the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, Government of India letter No. 2154/PO/Re/S/22/CPCSEA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, D., Ali, S.A. On the Novel Process of Pristine Microplastic Bio-fragmentation by Zebrafish (Danio rerio). Arch Environ Contam Toxicol 84, 299–306 (2023). https://doi.org/10.1007/s00244-023-00987-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-023-00987-2


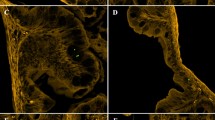



 showing cracks and holes caused by bio fragmentation
showing cracks and holes caused by bio fragmentation
