Abstract
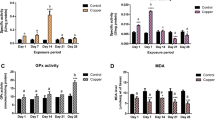
The in vivo toxic effects of sublethal treatment of 40 and 80% of 48-h LD50 of topically applied trace metals [copper (Cu), lead (Pb), and zinc (Zn)] on oxidative stress biomarkers in the digestive gland of Theba pisana were examined. Oxidative individual perturbations were assessed by measuring nonenzymatic (glutathione; GSH) and enzymatic (catalase, CAT; glutathione peroxidase, GPx; and glutathione-S-transferase, GST) antioxidants in digestive gland of the snails. Lipid peroxidation (LPO) was also evaluated as a marker of cell damage. The results indicated that the copper ion was the most potent metal against this snail, followed by zinc and lead, for which the corresponding LD50 values were 37.88, 261.72, and 652.55 μg/snail, respectively. The no-observed effect concentration (NOEC) values for Cu, Zn, and Pb were 10, 50, and 500 μg/snail, respectively, and the corresponding lowest-observed effect concentration (LOEC) values were 50, 100, and 1000 μg/snail. All trace metals resulted in a significant increase in the level of LPO, whereas a significant decline in the content of GSH was observed when compared with untreated controls. Treatment with both sublethal doses of the metals caused significant increase in CAT activity, except in the case of 40% LD50 Zn and 80% LD50 Cu, which exhibited no alteration in CAT when compared to control animals. GPx was significantly increased in snails exposed to 40% LD50 Cu and Pb as well as 80% LD50 Cu. However, an opposite effect was observed in snails exposed to 80% LD50 Pb and in either 40 or 80% LD50 of Zn-intoxicated animals. Treatment with Pb at two sublethal doses significantly increased GST activity, whereas treatment the animal with Cu caused significant inhibition in this enzyme. Snails exposed to 40% LD50 Zn showed significant enhancement of GST, whereas snails exposed to 80% LD50 showed ignificantly reduced GST activity. Biphasic responses were observed for CAT, GPx, and GST activities in snails exposed to Cu, Pb, and Zn, respectively. This study suggests that upregulation of the antioxidant enzyme activities, elevation of LPO, and the reduction in GSH content is related to oxidative stress in this species that could be useful as biomarkers for the evaluation of contaminated terrestrial ecosystems.





Similar content being viewed by others
References
Almeida EA, Miyamoto S, Bainy ACD, Medeiros MH, Mascio P (2004) Protective effect of phospholipid hydroperoxide glutathione peroxidase (PHGPx) against lipid peroxidation in mussels Perna perna exposed to different metals. Marine Pollut Bull 49:386–392
Beeby A, Richmond L (2002) Evaluating Helix aspersa as a sentinel for mapping metal pollution. Ecol Indicators 1:261–270
Beers RF Jr, Sizer IW (1952) Spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Berger B, Dallinger R (1993) Terrestrial snails as quantitative indicators of environmental metal pollution. Environ Monitor Assess 25:65–84
Bouskill NJ, Handya RD, Fordb TE, Galloway TS (2006) Differentiating copper and arsenic toxicity using biochemical biomarkers in Asellus aquaticus and Dreissena polymorpha. Ecotoxicol Environ Safety 65:342–349
Buchner F, Hofer R, Krumschnabel G, Doblander C (1993) Disturbances in the pro-oxidant-antioxidant balance in the liver of bullhead (Cottus gobio L.) exposed to treated paper mill effluents. Chemosphere 27:1329–1338
Canesi L, Viarengo A, Leonzio C, Filippelli M, Gallo G (1999) Heavy metals and glutathione metabolism in mussel tissues. Aquat Toxicol 46:67–76
Chandran R, Sivakumar AA, Mohandass S, Aruchami M (2005) Effect of cadmium and zinc on antioxidant enzyme activity in the gastropod, Achatina fulica. Comp Biochem Physiol C 140:422–426
Cheung SG, Tai KK, Leung CK, Siu YM (2002) Effects of heavy metals on the survival and feeding behaviour of the sandy scavenging gastropod Nassarius festivus (Powys). Marine Pollut Bull 45:107–113
Chiu DTY, Stults FH, Tappal AL (1976) Purification and properities of rat lung soluble glutathione peroxidase. Biochem Biophys Acta 445:558–566
Company R, Serafim A, Cosson RP, Fiala-Médioni A, Camus L, Colaço A, Serrão-Santosand R, Bebianno MJ (2008) Antioxidant biochemical responses to long-term copper exposure in Bathymodiolus azoricus from Menez–Gwen hydrothermal vent. Sci Total Environ 389:407–417
Costat program (1985) Costat user manual. Version 2. Cohort Software inc., Tucson, AZ
Cunha I, Mangas-Ramirez E, Guilhermino L (2007) Effects of copper and cadmium on cholinesterase and glutathione-transferase activities of two marine gastropods (Monodonta lineata and Nucella lapillus). Comp Biochem Physiol C 145:648–657
Cuypers A, Vangronsveld J, Clijsters H (1999) The chemical behaviour of heavy metals plays a prominent role in the induction of oxidative stress. Free Radical Res 31:39–43
Dallinger R (1994) Invertebrate organisms as biological indicators of heavy metal pollution. Appl Biochem Biotechnol 48:27–31
Dallinger R, Berger B, Triebskorn R, Köhler H (2002) Soil biology and ecotoxicology. In: Barker GM (ed) The biology of terrestrial molluscs. CAB International, Oxon, UK, pp 489–525
Doyotte A, Cossu C, Jacquin MC, Babut M, Vasseur P (1997) Antioxidant enzymes, glutathione and lipid peroxidation as relevant biomarkers of experimental or field exposure in the gills and the digestive gland of the freshwater bivalve Unio tumidus. Aquat Toxicol 39:93–110
Dunnett CW (1955) A multiple comparison procedure for comparing several treatments with a control. J Am Statist Assoc 50:1096–1121
Elia AC, Galarini R, Dorra AJM, Taticchi MI (2007) Heavy metal contamination and antioxidant response of a freshwater bryozoan (Lophopus crystallinus Pall., Phylactolaemata). Ecotoxicol Environ Safety 66:188–194
Escobar JA, Rubio MA, Lissi EA (1996) SOD and catalase inactivation by singlet oxygen and peroxyl radical. Free Radical Biol Med 20:285–297
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, London, p 318
Godan D (1983) Pest slugs and snails: Biology and control. Springer-Verlag, Berlin
Gomot A (1997) Dose-dependent effect of cadmium on the growth of snails in toxicity bioassays. Arch Environ Contam Toxicol 33:209–216
Gutteridge MC (1995) Lipid peroxidation and antioxidants as biomarker of tissue damage. Clin Chem 41:1819–1828
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, New York
Jamil K (2001) Bioindicators and biomarkers of environmental pollution and risk assessment. Science Publishers, Inc., Enfield, NH & Plymouth, UK
Laskowski R, Hopkin SP (1996) Accumulation of Zn, Cu, Pb and Cd in the garden snail (Helix aspersa): implications for predators. Environ Pollut 91(3):289–297
Livingstone DR, Lips F, Garcia MP, Pipe RK (1992) Antioxidant enzymes in the digestive gland of the common mussel Mytilus edulis. Marine Biol 112:265–276
Lowry OH, Rasebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Marigomez IA, Kortabitarte M, Dussart GBJ (1998) Tissue level biomarkers in sentinel slugs as cost-effective tools to assess metal pollution in soils. Arch Environ Contam Toxicol 34:167–176
McLoughlin N, Yin D, Maltby L, Wood RM, Yu H (2000) Evaluation of sensitivity and specificity of two crustacean biochemical biomarkers. Environ Toxicol Chem 19:2085–2092
Nair V, Turner GE (1984) The thiobarbituric acid test for lipid peroxidation structure of the adduct with malondialdehyde. Lipids 19:84–95
Nusetti O, Esclapes M, Salazar G, Nusetti S, Pulido S (2001) Biomarkers of oxidative stress in the polychaete Eurthoe complanata (Amphinomidae) under short-term copper exposure. Bull Environ Contam Toxicol 66:76–81
Orbea A, Fahimi HD, Cajaraville MP (2000) Immunolocalization of four antioxidant enzymes in digestive glands of mollusks and crustaceans and fish liver. Histochem Cell Biol 114:393–404
Owens WI, Belcher RV (1965) A colorimetric micro-method for the determination of glutathione. Biochem J 94:705–711
Pinto E, Sigaud-Kutner TCS, Leitao MAS, Okamoto OK, Morse D, Colepicolo P (2003) Heavy metal-induced oxidative stress in algae. J Phycol 39:1008–1018
Radwan MA, Essawy AE, Abdelmeguied NE, Hamed SS, Ahmed AE (2008) Biochemical and histochemical studies on the digestive gland of Eobania vermiculata snails treated with carbamate pesticides. Pesticide Biochem Physiol 90:154–167
Regoli F, Gorbi S, Fattorini D, Tedesco S, Notti A, Machella N, Bocchetti R, Benedetti M, Piva F (2006) Use of the land snail Helix aspersa as sentinel organism for monitoring ecotoxicologic effects of urban pollution: an integrated approach. Environ Health Perspect 114:63–69
Regoli F, Gorbi S, Frenzilli G, Nigro M, Corsi I, Forcardi S, Winston GW (2002) Oxidative stress in ecotoxicology: from the analysis of individual antioxidants to a more integrated approach. Marine Environ Res 54:419–423
Regoli F, Gorbi S, Machella N, Tedesco S, Benedetti M, Bocchetti R, Notti A, Fattorini D, Piva F, Principato G (2005) Prooxidant effects of extremely low frequency electromagnetic fields (ELF-EM) in the land snail Helix aspersa. Free Radical Biol Med 39:1620–1628
Regoli F, Hummel H, Amiard-Triquet C, Larroux C, Sukhotin A (1998) Trace metals and variations of antioxidant enzymes in Arctic bivalve populations. Arch Environ Contam Toxicol 35:594–601
Regoli F, Nigro M, Bertoli E, Principato G, Orlando E (1997) Defenses against oxidative stress in the Antarctic scallop Adamussium colbecki and effects of acute exposure to metals. Hydrobiologia 355:139–144
Regoli F, Principato G (1995) Glutathione, glutathione-dependent and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions: implications for the use of biochemical biomarkers. Aquat Toxicol 31:143–164
Roling JA, Baldwin WS (2006) Alterations in hepatic gene expression by trivalent chromium in Fundulus heteroclitus. Marine Environ Res 62:122–127
Rorke MA, Gardner DR (1974) Lethality and behavioural symptoms produced by some organophosphorous compounds in the snail (Helix aspersa). Bull Environ Contam Toxicol 11(5):417–424
Torres MA, Testa CP, Gaspari C, Masutti MB, Panitz CMN, Curi-Pedrosa R, Almeida EA, Di Mascio P, Wilhelm Filho D (2002) Oxidative stress in the mussel Mytella guyanensis from polluted mangroves on Santa Catarina Island, Brazil. Marine Pollut Bull 44:923–932
Triebskorn R, Henderson IF, Martin A, Köhler HR (1996) Slugs as target or non-target organisms for environmental chemicala. In: Symposium proceedings no. 66: slug and snail pests in agriculture, pp 65–72
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Safety 64:178–189
Verma RS, Mehta A, Srivastava N (2007) In vivo chlorpyrifos induced oxidative stress: attenuation by antioxidant vitamins. Pesticide Biochem Physiol 88:191–196
Vessey DA, Boyer TD (1984) Differential activation and inhibition of different forms of rat liver glutathione S-transferase by the herbicides 2,4-dichloro phenoxy acetate (2,4-D) and 2,4,S trichloro phenoxy acetate (2,4,S-T). Toxicol Appl Pharmacol 73:492–499
Viarengo A, Canesi L, Pertica M, Poli G, Moore MN, Orunesu M (1990) Heavy metal effects on lipid peroxidation in the tissues of Mytilus galloprovincialis Lam. Comp Biochem Physiol C 97:37–42
WHO/IPCS (1993) Environmental health criteria 155: biomarkers and risk assessment: concepts and principles. IPCS, World Health Organization, Geneva
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radwan, M.A., El-Gendy, K.S. & Gad, A.F. Oxidative Stress Biomarkers in the Digestive Gland of Theba pisana Exposed to Heavy Metals. Arch Environ Contam Toxicol 58, 828–835 (2010). https://doi.org/10.1007/s00244-009-9380-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-009-9380-1