Abstract
The pathogenesis of symptoms in urolithiasis is poorly understood. Traditionally increased endoluminal pressure is considered the main mechanism causing pain in the upper urinary tract but clinical data are sparse. The aim of the present study was to develop a new model related to mechanosensation in order to describe the geometric and mechanical properties of the renal pelvis in patients with kidney stone disease. Pressure measurement in the renal pelvis was done during CT-pyelography in 15 patients who underwent percutaneus nephrolithotomy. The sensory intensity was recorded at the thresholds for first sensation and for pain. 3D deformation and strain were calculated in five patients. The deformation of pelvis during distension was not uniform due to the complex geometry. The pelvis deformed to 113 ± 6% and 115 ± 11% in the longitudinal and circumferential directions, respectively. Endoluminal pressure in the renal pelvis corresponded positively to the sensory ratings but the referred pain area was diffuse located and varied in size. The present study provides a method for describing the mechanosensory properties and 3D deformation of the complex renal pelvis geometry. Although there was a relation between pressure and pain score, the non-homogenous spatial strain distribution suggests that the 3D biomechanical properties of the renal pelvis are not reflected by simple estimates of tension based on pressure and volume.





Similar content being viewed by others
Notes
The curvature is a parameter of the geometrical property. The curvature of a curve is by definition the reciprocal of the radius of the osculating circle. The curvature is taken to be positive if the curve turns in the same direction as the surface’s chosen normal, and otherwise negative. The smaller curvature the flatter is the surface.
At each point P of a differentiable surface in 3D Euclidean space, a normal plane at P is one that contains the normal, and will therefore also contain a unique direction tangent to the surface and cut the surface in a plane curve. This curve will in general have different curvatures for different normal planes at P. The principal curvatures and second principal curvature at p are the maximum and minimum values of this curvature.
References
Pedersen KV, Drewes AM, Frimodt-Møller FC et al (2010) Visceral pain originating from the upper urinary tract. Urol Res 38:345–355
Ammons WS (1992) Renal afferent inputs to ascending spinal pathways. Am J Physiol 262:165–176
Gregersen H (2003) Gastrointestinal smooth muscle mechanical behaviour and neural circuits, Chap 5. In: Biomechanics of the gastrointestinal tract, Springer, Berlin, pp 137–180
Drewes AM, Gregersen H, Arendt-Nielsen L (2003) Experimental pain in gastroenterology. A reappraisal of human studies. Scand J Gastroenterol 38:1115
Fillinger MF, Raghaven ML, Marra SP et al (2002) In vivo analysis of mechanical wall stress and abdominal aortic aneurysm rupture risk. J Vasc Surg 36:589–597
O’Dell WG, McCulloch AD (2000) Imaging three-dimensional cardiac function. Annu Rev Biomed Eng 2:431–456
Liao D, Gregersen H, Hausken T et al (2004) Analysis of surface geometry of the human stomach using real-time 3-D ultrasonography in vivo. Neurogastroenterol Motil 16:315–324
Liao D, Duch BU, Stodkilde-Jorgensen H et al (2004) Tension and stress alculations in a 3-D Fourier model of gall bladder geometry obtained from MR images. Ann Biomed Eng 32:744–755
Shi P, Sinusas AJ, Constable RT et al (2000) Point-tracked quantitative analysis of left ventricular surface motion from 3-D image sequences. IEEE Trans Med Imaging 19:36–50
Papademetris X (2000) Estimation of 3D Left ventricular deformation from medical images using biomechanical models, Ph.D. Dissertation, Yale University, New Haven, CT. http://noodle.med.yale.edu/thesis, accessed May 2000
Papademetris X, Sinusas AJ, Dione DP et al (2001) Estimation of 3D left ventricular deformation from echocardiography. Med Image Anal 5:17–28
Kopp UC, Smith LA, Pence L (1994) Na+–K+ ATPase inhibition sensitizes renal mechanoreceptors activated by increases in renal pelvic pressure. Am J Physiol Regul Integr Comp Physiol 267:1109–1117
Page AJ, Martin CM, Blackshaw LA (2002) Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol 87:2095–2103
Phillips RJ, Powley TL (2000) Tension and stretch receptors in gastrointestinal smooth muscle: re-evalusting vagal mechanoreceptor eletrophysiology. Brain Res Rev 34:1–26
Djurhuus J C (1980) Aspects of renal pelvic function, Thesis; University of Copenhagen
Jung H, Nørby B, Frimodt-Møller PC et al (2008) Endoluminal isoproterenol irrigation decrease renal pelvic pressure during flexible ureterorenoscopy: a clinical randomized, controlled study. Eur J Urol 54:1404–1413
Auge BK, Pietrow PK, Lallas CD et al (2004) Ureteral access sheath provides protection against elevated renal pressures during routine flexible ureteroscopic stone manipulation. J Endourol 18:33–36
Risholm L (1954) Studies on renal colic and its treatment by posterior splanchnic block. Acta chir Scand suppl 184:1–64
Cervero F, Sann H (1989) Mechanically evoked responses of afferent fibres innervating the guinea-pigs ureter: an in vitro study. J Physiol 412:245–266
Arendt-Nielsen L, Laursen RJ, Drewes AM (2000) Referred pain as an indicator of neural plasticity. Prog Brain Res 129:343–356
Fung YC (1977) Analysis of strain, foundations of solid mechanics, Chap 4. Prentice-Hall international INC, Englewood Cliffs, pp 89–108
Acknowledgments
This study was supported by the Beckett Foundation and the Local Research Foundation of Fredericia and Kolding Hospitals. Thanks to Elena Steffensen, Department of Radiology, Aalborg Hospital, Aarhus University Hospital, Aalborg, Denmark, for helping with image segmentation. Thanks to Ole Graumann, Urological Research Center, Hospital Littlebelt, Fredericia, Denmark, for helping with figure creation.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Strain calculation
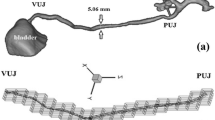
The membrane strain in every triangle plane can be defined with the displacement of every point on the surface and the coordinates of each three points that define a triangle determine a plane. The coordinates of these points in that plane at VAS = 1 are denoted as x i and y i (i = 1, 2, 3, are three points of the plane). The displacement in the local system of the points at VAS = 1 to their position at VAS = 5 are denoted as u i , v i and w i (u, v and w are, respectively, the displacement in x, y, and z directions, Fig. 6).
Schematic diagram of the global coordinate system XYZ and the local coordinate system xyz on one triangle face with nodes 1, 2, and 3 at VAS = 1. The displacement at the local coordinate system on the node 3 (u3, v3 and w3) is illustrated as an example. All strains were calculated based on the local coordinate system. For details about the transformation between the global coordinate system and the local coordinate system, see [21]
The displacements are assumed to be in a displacement field that is a linear function of position in the plane of the triangle. For example
This equation with known displacement and the position of three markers substituted for u i , x i and y i provides a set of three equations for the three coefficients, a 1, a 2 and a 3.
Similarly, the data provide the information required to determine the coefficients in the following equations
where \( a_{4} \cdots a_{9} \) are coefficients that could be defined with known displacement (v i and w i ) between surface of VAS = 1 and VAS = 5 and coordinates (x i and y i ) on surface of VAS = 1
The components of the Green strain in the plane are defined by the following equations
with the known coefficients \( a_{1} \cdots a_{9} \) and substituting Eqs. A1 and A2 into A3, the strain on the triangle plane can thus be obtained.
The principal strain and the corresponding principal orientation are calculated from:
where E 11, E 22 are principal strains and {l 1, m 1}T and {l 2, m 2}T are corresponding principal directions in the local coordinate system. There is no shear strain in the principal directions. T means transpose of the matrix or vectors.
The equivalent strain is defined as:
Rights and permissions
About this article
Cite this article
Pedersen, K.V., Liao, D., Osther, S.S. et al. Distension of the renal pelvis in kidney stone patients: sensory and biomechanical responses. Urol Res 40, 305–316 (2012). https://doi.org/10.1007/s00240-011-0425-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-011-0425-3