Abstract
Background
Bioprinting is one of the most rapidly developing fields in medicine. Plastic and reconstructive surgery will be affected enormously by bioprinting, due to its original purpose of restoring injured or lost tissue. This article in particular has the purpose to analyze the current state of bioprinted tissues as well as research engagement for its application in plastic and reconstructive surgery.
Material and methods
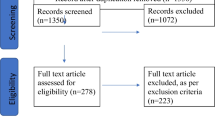
A systematic search for the time span between 2000 and 2022 was performed on EMBASE, PubMed, Scopus, and Web of Science databases according to the PRISMA Guidelines. Criteria for the selection of publications were in vitro, animal in vivo, and human in vivo studies where three-dimensional bioprinting of tissue was performed. We extracted data such as (a) author’s country of origin, (b) in vitro study, (c) animal in vivo study, and (d) human in vivo study and categorized the publications by topics such as (1) neural tissue, (2) vascularization, (3) skin, (4) cartilage, (5) bone, and (6) muscle. Additionally, recent discoveries of in vivo animal trials were summarized.
Results
Out of a pool of 1.629 articles, only 29 publications met our criteria. Of these publications, 97% were published by university institutions. Publications from China (28%, n=8), the USA (28%, n=8), and Germany (10%, n=3) led the publication list on 3D bioprinting. Concerning the publications, 45% (n=13) were in vitro studies, 52% (n=15) in vivo studies on animal models, and 3% (n=1) pilot clinical studies on humans as reported by Zhou et al. (EBioMedicine 28: 287–302, 2018). Regarding the classification of topics, our study revealed that publications were mainly in the field of 3D printing of cartilage (n=13, 39%), skin (n=7, 21%), bone (n=6, 18%), and vascularization (n=5, 15%).
Conclusions
To this date, it has not been yet possible to bioprint whole tissue systems. However, the progress in three-dimensional bioprinting is rapid. There are still some challenges, which need to be overcome regarding cell survival before and during the printing process, continuation of architecture of bioprinted multilinear cells, and long-term stabilization and survival of complex tissues.
Level of evidence: Not ratable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Charles Hull patented a stereolithography (SLA) three-dimensional printer in 1984 [1]. Since then, the possibilities of three-dimensional printing have evolved rapidly. Another milestone was reached in 2003 when Thomas Boland created the first bioprinting device using an HP-Inkjet Printer [2].
The different types of tissues in the human body are highly specialized, which is shown by their expectionally organized structures. Most are composed of multiple types of cells and extracellular matrix, as well as vascular and neural integration. But all tissues have different compositions, which can cause a challenge within the bioprinting process and design of the tissue. Cartilage tissue, for example, does not contain vascular or nervous systems, which is beneficial in bioprinting [3].
With rapid technological development, bioprinting experienced a revolution from being a futuristic vision to becoming an available approach within the medical field [4, 5]. There are many different possibilities for the clinical utilization of tissues produced by bioprinting [6]. The demand has risen rapidly over the past few years, especially in plastic and reconstructive surgery. One of the main reasons for this is the lack of donor organs/tissues in many countries across the world. Nevertheless, there is still a big debate about whether it is even possible to bioprint functioning and transplantable organs. Another important use for bioprinted, functional tissues will be the testing and evolving of new drugs [7]. There are a lot of potential advantages in this field such as reducing the number of laboratory animal testing and performing tests directly on targeted, three-dimensional tissue models resulting in better and more accurate conclusions.
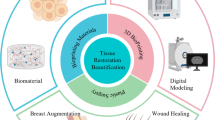
Preoperative 3D imaging plays a crucial role in the field of plastic surgery, particularly in the context of utilizing 3D bioprinting for procedures such as breast reconstruction. The integration of 3D imaging technology allows surgeons to plan and execute surgeries with greater precision, ultimately leading to more valuable and effective outcomes. When it comes to breast reconstruction, 3D imaging provides a comprehensive and detailed view of the patient’s anatomy. It allows surgeons to capture accurate measurements, identify asymmetries, and assess the unique characteristics of the patient’s breast tissue. This information is essential for creating personalized surgical plans and achieving natural-looking results. One of the main advantages of preoperative 3D imaging is its ability to facilitate the development of 3D artificial tissues using bioprinting techniques. By scanning the patient’s breast area, the imaging technology generates a digital 3D model that serves as a blueprint for the creation of custom implants or tissue scaffolds. These 3D models can be manipulated and modified to match the patient’s desired aesthetic outcome and address any specific anatomical considerations. Additionally, 3D imaging enables surgeons to simulate and visualize the surgical procedure before it is performed. By virtually manipulating the 3D model, surgeons can test different approaches, evaluate potential outcomes, and optimize the surgical plan. This allows for better communication and collaboration among the surgical team, ensuring that all stakeholders have a clear understanding of the intended surgical goals. The integration of 3D bioprinting with preoperative 3D imaging offers several benefits for both the surgeon and the patient. By using 3D artificial tissues, surgeons can create personalized implants or scaffolds that closely match the patient’s anatomy, leading to improved aesthetics and functionality. The ability to plan and simulate surgeries using 3D imaging reduces the risk of complications and allows for more predictable outcomes. Furthermore, this approach enables a more efficient use of surgical resources and may shorten the overall surgical time [8,9,10].
The medical specialty of plastic and reconstructive surgery is already important for further development of bioprinting of human tissues and will be even more so in the future [11]. The main reason is the purpose of plastic and reconstructive surgery, to restore and reconstruct the human body [12,13,14]. Especially in burn surgery, bioprinted skin can offer a huge benefit [15]. In cases of severe burn injuries, a lack of donor skin is a challenge to overcome. The primary aim of bioprinting in these cases will be the replacement of skin grafts with a satisfactory amount of bioprinted skin.
Current bioprinting technologies can be divided into different techniques. The most common ones are droplet-based (DBB), extrusion-based (EBB), and laser-based bioprinting (LBB) [16] (Fig. 1).
Droplet-based bioprinting was originally developed using inkjet printers. Cells and biomaterials are put into shape in form of droplets. The moving head is therefore able to form a specific three-dimensional structure. As shown in Fig. 1, different techniques of ejection have been developed. Thermal or heater-based printers form a vapor bubble, which eventually collapses and provides pressure, thus ejecting the bioink containing cells and biomaterials.
Piezoelectric-based printers form an acoustic wave after voltage is applied. This results in forming many droplets of bioink, which are dropped on the substrate. Today droplet-based printers are mainly used to form small scaffolds. To increase the printing speed, inkjet printers with multiple heads have been developed. Another recent innovation was the development of microvascular multinozzle printheads.
In extrusion bioprinting, the bioink is extruded consistently from a valve, in form of a filament. This process is driven by pneumatic, piston or screw-like forces [17]. Extrusion bioprinting stipulates different properties from the bioink. It needs to be fluid enough to be extruded through the nozzle, while stable enough to keep its shape during the printing process. Extrusion or micro-extrusion bioprinting is able to form a high viscous cell substrate. Though a disadvantage is the relatively low resolution compared to either droplet-based or laser-based bioprinting.
Laser-based bioprinting uses a laser to eject droplets onto a receiver substrate. The donor layer is supported by a “ribbon” which absorbs the energy from the laser pulse. The laser generates energy, ejecting a droplet from a donor layer onto the receiving substrate. There is no nozzle system in LBB, a construction detail that eliminates clogging, which happens in droplet-based and extrusion-based bioprinting. Therefore, LBB offers the opportunity to print high-viscose bioinks. However, the cell viability is lower due to cell death by laser-energy [18].
The bioink in the process consists of biomaterial and living cells. The synchronized deposition of said bioink generates the 3D constructs of tissue. Bioinks are able to meet the requirements of living cells before, during, and even after the printing process. Cells need sufficient oxygen supply and nutrition (proteins, lipids, and carbohydrates etc.), as well as vitamins and minerals which may be able to diffuse through the bioink. Another challenge is creating enough space for cell migration, proliferation, and creation of extracellular matrix.
This article has the purpose to analyze the current state of research concerning bioprinted tissues and research engagement for application in plastic and reconstructive surgery. Additionally, recent discoveries of in vivo animal trials were summarized.
Material and methods
A systematic search for the time span between 2000 and 2022 was performed on EMBASE, Pubmed, Scopus, and Web of Science databases according to the PRISMA guidelines. Inclusion criteria for the selection of publications were in vitro, animal in vivo, and human in vivo studies where three-dimensional bioprinting of tissue was used for plastic and reconstructive surgery purposes.
We extracted data such as (a) author’s country of origin, (b) in vitro, (c) animal in vivo, and (d) human in vivo study and sorted the publications by topics, such as (1) nerve tissue, (2) vascularization, (3) skin, (4) cartilage, (5) bone, and (6) muscle. All publications regarding 3D bioprinting for plastic and reconstructive surgery purposes were classified in abovementioned topics and recent discoveries of in vivo animal trials were summarized. A descriptive statistical analysis was conducted.
An ethics vote was not necessary for the conduct of the study.
Results
Out of 1.629 articles, only 29 publications met our criteria and were therefore included in our study. Of these publications, 97% came from university institutions. Research from China (28%, n=8), the USA (28%, n=8), and Germany (10%, n=3) led the publications on bioprinting. Of the publications, 45% (n=13) were in vitro studies, 52% (n=15) in vivo studies on animal models, and 3% (n=1) pilot clinical studies on humans [19]. Regarding the classification of topics, our study revealed that the publications were mainly in the field of three-dimensional bioprinting of cartilage (n=13, 39%). Figure 2 shows the distribution of other topics such as skin (n=7, 21%), bone (n=6, 18%), and vascularization (n=5, 15%).
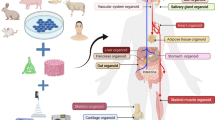
For plastic and reconstructive surgery, many tissues are of great importance. The following section introduces and critically reviews skin, muscle, cartilage, bone, vascular, neural, and adipose tissues, in terms of the most advanced in vivo studies.
Skin tissue
The human skin consists of three layers (epidermis, dermis, hypodermis). The epidermis consists of keratinocytes, melanocytes, Langerhans’ cells, and Merkel’s cells. The dermis houses sweat glands, hair, hair follicles, muscles, sensory neurons, and blood vessels. Cubo et al. showed in 2016 [20] in vitro and in vivo use of bioprinted skin. They were able to design human, bi-layered skin using bioinks containing human plasma as well as primary human fibroblasts (hFBs) and keratinocytes (hKCs) that were obtained from skin biopsies. The approach of this group was the printing of two layers, with the lower one made of fibrin and hFBs, while the upper layer was made mostly from keratinocytes. After the bioprinting process, the assays were grafted onto nude immunodeficient mice. Full-thickness circular wounds of 12 mm in diameter were produced by means of a punch biopsy on the dorsum of each mouse. A circular sample of the same diameter was obtained by the same biopsy from the printed skin substitutes and placed on the generated wounds and covered by the skin which previously has been removed from these mice, devitalized by three cycles of freezing and thawing. The devitalized skin was kept in place with the help of sutures. The grafts were analyzed 8 weeks after this procedure took place. The histological analysis proved the similarity of the printed and grafted skin compared to the original human skin. After a more detailed examination, using immunofluorescence, a proliferative basal membrane has been identified. The correct formation of the dermo-epidermal junction of the skin was confirmed by labeling with an antibody against human collagen VII, which is very important for the stability of human skin. Adding the fact of the short printing time of only 35 min, this method shows the possibility of bioprinting skin for therapeutic or industrial purposes in an automatized manner.
The laboratory of the Wake Forest Institute also works on bioprinted skin tissue. They published an article about their research regarding in situ bioprinting in 2019 [21]. In their study, keratinocytes and fibroblasts have been printed directly into a dermal defect zone in athymic mice. Their results showed improved healing with bioprinted wound coverage compared to untreated mice with the same dermal defect zone. This study has shown the possibility to treat dermal defects directly with an inkjet printer. They compared the results to cell spraying techniques, which bring a mixture of cells in an unsorted manner into the wound. The bioprinting approach carries the fibroblasts and keratinocytes in a layer-by-layer manner into the wound area, matching the structure of human skin.
The laboratory of the Wake Forest Institute published another trial in 2020. Contrary to their previous trial, which contained often only a few different cell components, six different human cell types were used to recreate the specific skin layers: epidermis—human keratinocytes and human dark-melanocytes (ratio—9:1); dermis—human dermal fibroblasts, human follicle dermal papilla cells, and human dermal microvascular endothelial cells (HDMECs; ratio 6:1:1); and hypodermis human preadipocytes. All cells were derived from humans, isolated and cultured. Using an extrusion-based bioprinting system, they developed themselves. Three layers of a cell-laden fibrin bioink to form a 3 × 3-cm-wide skin graft were printed. First, a bottom hypodermal layer, containing the preadipocytes, was printed. It was followed by a second dermal layer, containing human dermal fibroblast, human follicle dermal papilla cells, and human dermal microvascular endothelial cells. On top, an epidermal layer, containing human keratinocytes and human dark-melanocytes, was created. A surgically established wound defect in athymic mice was covered with the bioprinted skin. The results were evaluated after 21 days using histological, immunofluorescence, and electronic microscopy. This study showed bioprinted skin being able to function as an epidermal wall and inducing regrowth of a normal collagen ECM architecture. The cells in the bioprinted tissue laid down a healthy, basket-woven collagen network, proofed by Picrosirius red collagen fiber image analysis. Overall, this study is the first one to promote collagen remodeling in a bioprinted construct. An increased epidermal coverage (proofed via histological staining) compared to untreated wounds was found. The authors stated, the approach could be improved to include additional cell types. The included melanocytes did not lead to significant pigmentation after 21 days. There were also no human hair follicles shown on day 21.
The authors limited their trial as it featured only one time point. Earlier time points might be of interest in terms of acute immune response and early wound epithelization. A longtime point might be of interest regarding scars and wound contractions [12]. Yanez et al. bioprinted a bi-layered tissue construct containing neonatal human dermal fibroblasts (NHDF), human dermal microvascular endothelial cells (HDMECs), and neo-natal human epidermal keratinocytes (NHEK). A solution of NHDFs and collagen was pipetted onto a glass slide, working as a dermal layer. For the printing process, HDMECs were mixed with a thrombin solution. The epidermal layer was created by pipetting a NHEK-collagen solution. The mice underwent surgery in which a skin defect on their back was established. The results showed a lesser wound contraction of up to 17% using the bioprinted constructs compared to natural wound healing capacities. The histological results showed a bi-layered construct close to natural human skin. Immunofluorescence showed the presence of bioprinted human cells 4 weeks post-implantation. The authors state that adding endothelial cells to the bioprinted constructs would be the first possible improvement [22].
Abaci et al. [23] published an article in 2018, where they added human hair follicles into human skin constructs. The in vivo line of their trial involved human skin constructs seeded with hair follicles. The first step was the formation of a dermal layer using mostly fibroblasts. The printed construct contained microwells. They then seeded dermal papilla cells at the bottom and human keratinocytes on top. To form hair follicles, keratinocytes were treated with Lef-1, which promotes the differentiation into hair follicle cells. They were able to seed the hair follicles within the human skin construct with a density of 255/cm2, which comes very close to hair density on the human scalp. To secure vascularization of the construct, the dermis was treated with human umbilical vein endothelial cells, together with the dermal fibroblasts. They were afterwards transplanted onto nude mice. After 4 weeks, substantial hair growth from the grafts could be observed. Immunofluorescence using K71 (inner root sheath markers) and VCAN (versican) showed human nuclei in the hair follicles. PCR revealed that the growing hair was built from human cells. The authors state, their method has the advantage of planned formation of hair follicles due to the use of Lef-1, while other trials had to wait for spontaneous formation of hair follicles. Their ability to vascularize the hair follicles is a huge step forward regarding the possible treatment of severe skin loss. The authors state, their ability to create up to 15 million units of dermal papilla cells from a very small donor site (0.5 cm2) results in more than 5000 hair follicles, making it very feasible and efficient in hair restoration therapy.
Muscle tissue
The defining attribute of muscle tissue is the ability to contract and consequently perform a specific motion. For this function, the cells need to be connected in a certain way to work as a functional syncytium. Due to their high demand for energy, the myocytes necessarily need a sufficient blood supply. In reconstructive surgery, muscles are often used to close devastating wounds due to cancer, trauma, or infection [24, 25]. Sadly, utilizing muscles in surgical preparations for the human body are challenging to harvest. Therefore, bioprinted muscle tissue will have a huge impact in the field ofreconstructive surgery [26].
The Wake Forest Institute printed muscle tissue with the size of 15 mm × 5 mm × 1 mm from mouse myoblasts. The printed structures contained muscle fiber-like bundles with approximately 400-μm width. After 7 days, the structures were implanted onto nude rats. The peroneal nerve was embedded within the implanted structure to promote integration and innervation. After 2 weeks, the structures showed acetylcholine receptors, as well as vascularization tested by electromyography. The muscle action potential was tested and proved the response of the implanted structures to electrical stimulation [21]. The same group kept working on their principles in a new trial. The purpose of this new trial was to investigate, whether critical muscle loss could be restored by printed muscle tissue. The printed muscle tissue construct was composed of human primary muscle progenitor cells (hMPCs), which were biopsied from humans. The printing process included three components: the bioink containing the hMPCs, the sacrificial acellular bioink, and the supporting PCL pillar. Nonetheless, establishing vascular structures has not been possible to gain. To overcome the abovementioned problem, microchannels were created every 200 μm to ensure nutrition and oxygen supply. To test the printed muscle constructs under in vivo conditions, implantation in athymic mice followed. A defect zone of 30–40% of the size of the tibialis anterior muscle in combination with ablation of extensor digitorum longus (EDL) and extensor hallucis longus (EHL) muscles was created by surgery. These defects generate irreversible deficiencies, if stay untreated. The in vivo functional analysis was performed by measuring tetanic muscle force of the muscle with peroneal nerve stimulation. The bioprinted implant showed not only the maintenance of its original muscle volume during the 8-week period, but also a significant increase of the tetanic muscle force and tibialis anterior muscle weight. Overall, the in vivo study showed an 82% recovery of the initial tibialis anterior muscle’s function 8 weeks after implantation. The histological results showed vascularization and neural integration in the bioprinted constructs. These results show that 3D bioprinted muscle constructions can develop functionality by integration within the host’s vascular and nervous systems following implantation in the TA muscle defect. These 3D bioprinted skeletal muscles present a possible therapeutic approach for devastating muscle defect injuries.
Cartilage tissue
Defects of cartilage are observed in several diseases such as arthrosis, rheumatism, and injuries. The current therapeutic approaches are not fully satisfying in many cases, which makes bioprinting of cartilage very interesting not only for researchers but also for clinicians. Due to the histological structure of cartilage, a major challenge of bioprinting can be avoided. Cartilage tissue is neither vascularized nor innervated. The nutrition of the chondrocytes is provided via diffusion from adjacent tissues. This might be a reason why 3D printing of cartilage seems to be more advanced compared to other tissues. Nevertheless, there are still essential differences between bioprinted and native cartilage. Human cartilage can be histologically divided into hyaline cartilage, elastic cartilage, and fibrocartilage. Hyaline cartilage is mostly found in areas with high pressure such as joints, whereas elastic cartilage is found, e.g., in the Auricula Auris, Epiglottis, and Ligamenta flava. Fibrocartilage is found in spinal discs and menisci.
When transplanting cartilage for the reconstruction of organs, such as the ears or noses, it is necessary to ensure the diffusion of nutrients from the adjacent skin into the transplanted cartilage. This could be achieved by preforming grafts that already combine these two tissues. Apelgren et al. [27] achieved this by implanting cartilage grafts covered with full-thickness skin into nude mice. They used a combination of human bone marrow-derived mesenchymal stem cells (hBM-MSCs) and human nasal chondrocytes (hNCs) in combination with a cellulose/alginate bioink as cartilage. Via an extrusion printing process, they built 10×10×1.2-mm-wide grids, which were implanted into subcutaneous tissue in nude mice subsequently. After a healing period of 45 days, the subcutaneous pockets were opened again and full-thickness skin grafts from donor mice were added onto the cartilage grids. After another 60 days, half of the mice were euthanized, and the grafts were explanted. In the other group, the most upper layer of the skin pocket was removed and the surrounding skin was sutured to the full-thickness skin graft. After another 75 days, this group was euthanized as well and the preformed grafts were removed and fixated. No transplant failure was noted in neither of the groups. The histological observation showed a good incorporation of the implants without any sign of necrosis or detachment. However, lymphocytic invasion was observed. All in all, this study showed 3D printed cartilage tissue covered with skin being able to perform as a graft in situ. These constructs could be a possible treatment for defects of ears or noses. Diffusion of nutrients and oxygen, as well as cell proliferation, can even be enhanced by integrating microchannels or nanotubes into the scaffolds.
Kang et al. [28] printed a CT-based model of a human auricle with rabbit ear chondrocytes embedded in composite hydrogel consisting of gelatin and fibrinogen. After printing the ear-shaped constructs, the cell viability was as high as 91% on day 1. Some of the constructs were provided with microchannels. The microchannel-containing constructs showed enhanced tissue formation and more viable cartilaginous matrix compared to the constructs without microchannels. The ear constructs were also tested in vivo by implantation onto athymic mice. They were retrieved after 1 and 2 months, respectively. Histological observation revealed the new formed cartilage to be similar to native cartilage, with a glycosaminoglycan content reaching 20% of the GAG content in native ears. This trial proves the benefits of microchannels for the nutrition of printed cartilage tissue. However, it did not feature hyaline cartilage. According to the high prevalence of especially age-related joint dysfunctions, therapies for reconstruction of hyaline cartilage might even have the highest clinical relevance compared to other cartilage tissues. This might be the reason, why many researchers focus on hyaline cartilage.
Similarly, Shim et al. [29] printed osteochondral constructs using human turbinate-derived mesenchymal stromal cells (hTMSCs) that were isolated from three young male patients. The printing process started with the creation of a biodegradable PCL framework. The bioprinted construct was about 5-mm high and had a diameter of 5 mm. To reach the most realistic situation, the cylinder was divided into two distinct layers. The lower one was supposed to function as the subchondral layer and therefore a bioink containing hTMSCs, atelocollagen, and the osteogenetic factor rhBMP-2 was used. The structure was printed into the PCL framework until a height of 4 mm was reached. The superficial cartilage was printed using a bioink containing hTMSCs and chondrogenic factor TGF-β. This procedure shows the possibility to bioprint two-layered tissue consisting of bone and cartilage as found in joints.
For the in vivo trial, adult New Zealand rabbits were used. Therefore, a full-thickness osteochondral defect (5 mm in diameter and 5 mm in depth) penetrating the subchondral bone plate was created in the femoro-patellar groove. The bioprinted constructs were then implanted into the defect zone. After 8 weeks, the rabbits were euthanized and the constructs investigated. The defect was fully covered with neo-tissue. Additionally, the newly generated tissue exhibited a smooth surface, and the cartilage-like neo-tissue was lighter in color than the adjacent native cartilage. The histological and immunofluorescent evaluation also showed a good integration and viability.
All these results show very promising attempts of osteochondral rehabilitation but so far have all been tested on small animals only. Human articular cartilage is constantly exposed to pressure that is much higher than in mice, rabbits, or rats. This is why big animal trials may better mimic the actual circumstances in human joints. Unfortunately, big animal trials show less optimistic data. A group in Utrecht [30] conducted a big animal trial on Shetland ponies. Since mammalian cartilage consists of different zones, they compared the performance of a zonal-formed bioprinted cartilage construct with a non-zonal one. They surgically established a cartilage defect in the stifle joint of the ponies. Afterwards, they implanted a PCL bone anchor, whose fibers protruded into the artificial cartilage zone on top of the anchor. The cartilage consisted of two zones. These two zones were bioprinted in a thiolene cross-linkable hyaluronic acid/poly(glycidol) hybrid hydrogel (HA-SH/P(AGE-co-G)). The top zone contained articular cartilage progenitor cells (ACPCs) and the bottom zone contained mesenchymal stromal cells (MSCs). The defects of the control group were homogeneously filled with MSCs on top of the bone anchor. After a healing period of 6 months, the implanted constructs were investigated histologically. In both groups, no significant production of cartilage-like tissue has been found. However, the zonal constructs were stiffer and could withstand more pressure (147.5 ± 40.7 kPa) than the control group (96.9 ± 33.0 kPa, p < 0.05). Even though the difference between these two groups is significant, native cartilage tissue of the same animals ranged at 495.9 ± 174.0 kPa. Thus, the functional outcome of the printed constructs is by far not as high as the native tissue. Within this approach, many factors could relate to less quality of bioprinted constructs on which the loss of implanted cells and the early degradation of hydrogel could have the biggest impact.
Bone tissue
Bone tissue builds the very framework of the human body. The main function of bones is to protect the organs, produce blood cells, and store minerals. It is also essential for body movement. Bones come in many different shapes and sizes, which depend on their specific localization and function.. The regeneration of bone tissue with the support of 3D printed scaffolds has already found its way into treatment. The advantage of personalized prosthesis lies within the possibility to match the scaffold or prosthesis individually to the patient.
Another approach is the adjustment of scaffold porosity, such as three-dimensional printing enables to. Therefore, implants with variable mechanical properties can be brought into different regions of the body matching the respective requirements. Today’s approach features living cells, inducing migration, and proliferation of osteoblasts [31]. The Wake Forest Institute published an in vivo trial using human amniotic fluid-derived stem cells (hAFSCs) to rise up to osteogenic lineages. They were mixed with PCL (supporting polycaptrolactone polymer)/TCP (tricalcium phosphate). The printed constructs were implanted into a calvarial bone defect region of Sprague Dawley rats. They were analyzed 5 months later. The histological observation showed newly formed and vascularized bone tissue throughout the implants including the central region. No necrosis was shown in the constructs. In a control group of rats, which only received the cell-free scaffolds fibrotic tissue, ingrowth was observed and limited new bone formation was found in regions close to the periphery only. The authors state that they did not observe any immune response from the host, as well as the need for longer trials to observe the regeneration of bioprinted constructs [32].
Adipose tissue
The most important attribute of adipose tissue is its ability to store high amounts of lipids. The regulation of this function underlies complex hormonal mechanisms, which makes it difficult to bioprint a tissue that functions as its natural counterpart.
For bioprinting adipose tissue, human adipose-derived stem cells (hASCs) were utilized to create a bioink. The printed adipose tissue was used in an in vivo trial [33]. In this trial, they implanted the printed constructs with a size of 10 × 5 mm subcutaneously into nude mice. After 2 weeks of vascularization, a type IV collagenous structure was histologically detectable, demonstrating a successful tissue remodeling.
Neural/nervous tissue
To our knowledge, there are no trials regarding in vivo experiments with bioprinted neural tissue. However, research focuses on scaffold printing, helping damaged peripheral nerves to regrow and heal.
Discussion
Currently, bioprinting of whole tissue systems or even organs is still not possible. The translation of recent advances of animal in vivo studies to human in vivo experiments is still in development. However, the progress in bioprinting is growing rapidly. There are still some challenges to overcome. For bioprinting, the cells need to stay alive before and during the printing process, which demonstrates the importance of bioink’s utilization. Even after printing, a challenge will be to keep different types of cells, like specific tissue cells, cells from the vascular system or the connective tissue all at their wanted location and also ensuring their survival. All these different cells might have different requirements concerning the bioink. One general challenge is to obtain the highest possible biomimicry in bioprinted constructs. The term biomimicry means the imitation of nature in bioengineering. Due to the challenge of replicating the tissue on a microscale basis, researchers have found the utilization of growth factors to induce vascularization and innervation of the bioprinted construct.
Skin tissue
Regarding the development of bioprinted skin tissue, there are different approaches regarding skin’s formation. Most of them use a combination of keratinocytes and fibroblasts, mimicking an epidermal (keratinocytes) and dermal (fibroblasts) layer. All studies relied on integration of vessels after implantation. All trials showed the formation of human-like skin regarding the layered structure. All trials lack a sufficient longtime evaluation of the bioprinted constructs, regarding scarring and wound contraction, as an early investigation of the acute immune response after implantation.
In general, mouse or rat models would need to be transferred into porcine models in order to investigate their immune responses after implantation or in situ bioprinting. The trial by Jorgensen et al. included additional melanocytes and human hair follicles to fibroblasts and keratinocytes. However, the results showed no integration of these cell types regarding their function. The construction of bi-layered skin in the form of epidermis and dermis seems to work reliably and is assessed by many research groups. Contrarily, very promising results were achieved by Abaci et al. with forming vascularized hair follicles, using the combination of human umbilical vascular endothelial cells. However, small animal models sometimes differ from big animal models; therefore, the promising results should be taken carefully. The need for big animal models and finally human trials is clearly visible. The future possibilities of bioprinted skin constructs will affect burn treatment hugely and better the quality of life of burn victims or general victims of major skin loss [22].
Muscle tissue
The Wake Forrest Institute is very advanced in bioprinting methods; their constructs integrate very well into the host system. The myofiber-like structure of the bioprinted constructs seems to be working well, resulting in a restoration of muscle force. The measurement was done via peroneal nerve stimulation, which showed a significantly higher restoration compared to the control group.
Other measurements, like the gait pattern, were not observed in this study. They might be of higher importance in future approaches. In this trial, they showed vascular and neural integration. In the future, bioprinted autologous muscle tissues need to be investigated regarding host response, inflammatory response, and the regeneration processes generally, since immunocompromised animals were used in this trial.
In general, the bioprinting process of major muscle volume requires a vascular integration as early as in the bioprinting process, as well as a neural network. Therefore, neural components like neural cells, neurotrophins, or neurotransmitters might be advantageous to establish faster muscle recovery. Nonetheless, the bioprinted construct showed a similar structure to native muscles in form of multilayered myofiber bundles, leading a very promising path into the future. After implantation, vascularization, and innervation of the muscle construct, no major longtime loss of muscle recovery suppose be expected. Limitations regarding this approach that need to be overcome include among others increasing the possible size to restore larger muscle losses in the human body.
Cartilage tissue
The natural characteristics of mammalian cartilage, such as the lack of vascularization and innervation, make this tissue feasible for bioprinting approaches. The bioprinting process does not have to account vessels or nerves, which in general is one of the biggest challenges of this technology.
Nevertheless, the printed cartilage has to be embedded in an environment that ensures the diffusion of nutrients and oxygen into the cartilage. Therefore, it might be necessary to perform constructs that already combine cartilage and the nutrient providing tissue as Apelgren et al. [27] have shown. Doing so, organs like external ears or noses could already be reconstructed. The patient could come for an outpatient appointment, in which scanning of the contralateral auricle, harvesting of autologous cells, mixing with bioink, printing, and then bioincubating the construct subcutaneously on the forearm or on the abdomen could be conducted. The second visit could include the surgery and transplantation of the construct with its vessels (for example, radial artery and vein after initial implantation into the forearm) to the defect site with microsurgical anastomosis to the regional vessels. Therefore the possibility of bioprinting constructs for reconstruction of tissue defects is already given. This example may also work as an inspiration for future approaches regarding other tissues. It was also shown that the microstructure of the extracellular matrix/bioink can affect the rate of viable chondrocytes. The integration of microchannels, for instance, seems to lead to a better diffusion situation although the formation of microchannels cannot be found in natural cartilage tissue. Thus, they might have some disadvantages, like less stability and withhold against high pressures.
In hyaline cartilage, which occurs in joints, the functional units between cartilage and bone tissue are very important and need to be considered in the bioprinting process. These functional units need to withstand high pressures while the joint moves. The trial of Shim et al. showed the possibility of restoring a combined cartilage/bone defect without a significant host immune response in rabbits. However, a big animal trial by Mancini et al. showed fewer positive data. Experiments with big animals might be more realistic, since the forces in their joints are closer to those in human joints. The trials also show the treatment of a man-made established defect with a bioprinted construct of the same size. This is not the case in clinical situations and therefore needs to be addressed in the future [33].
Bone tissue
Bone tissue has been the focus of research for a very long time, mostly done by orthopedic surgeons. Currently, the approach of coated scaffolds, which induce the migration and proliferation of cells, is explored in human trials in cases of devastating bone loss. Bioprinting of bone tissue will become a very interesting approach not only for plastic and reconstructive surgeons. In cases of an urgently needed stabilization of bone structures, e.g., after spinal injuries, the implantation of viable bone might increase the chances of a positive outcome.
The Wake Forest Institute, once again, successfully implanted bioprinted bone tissue into Sprague Dawley rats. The migration of vascular systems into the bioprinted constructs shows the acceptance by the host. This capacity will be essential in future approaches of bigger constructs. The next steps might be the implementation of bioprinted tissue into bone defects in more intensively utilized regions like the femoral bone. For this to happen, the next achievements in the bioprinting process of bone tissue should be the implementation of an already established vascular system, as the maximum distance nutrition distance is about 200 μm [34]. In general, the trial showed the formation of vascularization and therefore has the potential to form tissue with clinically relevant size after improvement. Eventually, trials on humans might offer the chance to move the borders of modern medicine.
Adipose tissue
The results of the study published by Pati et al. [32] are very promising for plastic and reconstructive surgery as well as breast surgery. The cure of breast cancer is one of the great challenges of modern medicine. In the future, the bioprinting of adipose tissue in combination with a nipple/areola complex will enable to restore the psychosexual well-being of the former cancer patient. The next steps will be the formation of bigger adipose constructs and an implemented vascular system in combination with a defined artery and vein of the construct, but this will not be possible in the too distant future. With these conditions, the establishment of a “breast flap” can start in an animal trial and move on to human trials eventually.
Neural tissue
The development of functional bioprinted neural tissue is still a huge effort. Today, the best way of nerval transplantation is surgery by a skilled surgeon. In many cases of free flaps, the neural innervation will be lost after the transfer. Future work will focus on the possible bioprinting of neural tissue, mostly already embedded in other tissues, like muscle for motoric nerves or skin for sensitive nerves.
In all bioprinted tissue constructs, the vascularization to ensure its oxygenation and nutrition [35] is a biological challenge. The combination of specific tissue cells with connecting cells or stabilization factors, like collagen, will be of great challenge to ensure the stability and durability of the printed tissue. However, it can be part of specific bioinks or even printed cells might be able to produce specific tissue proteins, if needed. Some introduced trials showed the possibility of bioprinting tissues combined with specific growth-factors [23]. This enables the bioprinted construct to form a vascularization of its own. This approach seems to be very promising, but might be limited to skin tissue, or smaller constructs in other tissues. The innervation or neural integration of bioprinted constructs is of high relevance [36]. This is crucial for muscle tissue in order to restore its original function; additionally, it plays a considerable role in skin tissue. To our knowledge, there are no trials yet that tried to induce neural integration with growth factors or stem cells and growth factors. Another requirement is that of the hardware, which requests a lot of work from the 3D printers.
At first, the cell resolution needs to be optimized with a lower cell spacing than 100 μm. The printing speed and the scalability will be of great challenge in the future as well. The combination of high-speed printing and high resolution will result in higher shear forces. In order to reduce shear forces, the printing speed has to decrease, which brings the challenge of possible cell death within the printing process of higher volumes needed in clinical use. Another big challenge will be faced on the regulatory level. As mentioned above, stem cells are often used within the bioprinting process. In Europe, the use of stem cells is highly restricted by organs of state for ethical reasons. There is a big safety concern regarding the use of autologous stem cells. It has to be ensured that the manipulation of cells will not result in devolution, for example, a tumor.
This process might be referred to as 4D bioprinting. This term means the use of stimuli-responsive materials to bioprint constructs [37]. Some of the introduced trials used stem cells or growth factors to change the bioprinted construct within the process. This offers huge possibilities and enables to achieve a better biomimicry. Also, the growing importance of 4D printing in plastic surgery and future perspective take an important role. 4D printing is an emerging technology that has the potential to revolutionize various fields, including plastic surgery. Unlike traditional 3D printing, which involves creating static three-dimensional objects, 4D printing adds an additional dimension of time, allowing printed objects to change shape or function over time in response to external stimuli. This dynamic nature of 4D printing opens up new possibilities in the field of plastic surgery. In plastic surgery, the goal is to restore or enhance both the form and function of various body parts. 4D printing offers several advantages in achieving these goals. One of the key applications of 4D printing in plastic surgery is the development of dynamic implants. These implants can be designed to respond to the body’s natural movements or physiological changes. For example, in breast reconstruction, 4D-printed implants can mimic the behavior of natural breast tissue, expanding and contracting in response to body movements. This leads to more natural-looking and functional results. Another area where 4D printing holds promise is in the creation of tissue scaffolds for tissue engineering and regenerative medicine. These scaffolds can be printed using bioactive materials and designed to gradually degrade or change shape over time, promoting the regeneration of damaged or lost tissue. For instance, in facial reconstruction, 4D-printed scaffolds can be used to support the growth and regeneration of new tissue, enabling the restoration of facial features with improved aesthetics and functionality. Furthermore, 4D printing allows for the integration of smart materials and stimuli-responsive components into surgical implants or devices. These materials can be designed to respond to specific triggers such as temperature, pH, or light, enabling controlled and targeted drug delivery, wound healing, or tissue regeneration. Such advancements have the potential to enhance the outcomes of plastic surgery procedures and improve patient recovery. Looking to the future, 4D printing in plastic surgery holds exciting prospects. Researchers are exploring the use of advanced biomaterials and bioinks that can closely mimic the properties of natural tissues, providing better biocompatibility and integration with the body. They are also working on developing more sophisticated printing techniques, including multimaterial and multiaxis printing, to create complex structures and functional gradients. In addition, the integration of 4D printing with other technologies such as 3D bioprinting, stem cell research, and tissue engineering holds great promise. By combining these approaches, it may be possible to create patient-specific 4D bioprinted tissues and organs, allowing for complex reconstructions or replacements in plastic surgery. However, it is important to note that 4D printing in plastic surgery is still in its early stages, and there are challenges to overcome. These include the development of suitable materials with appropriate mechanical properties, biocompatibility, and long-term stability. There is also a need for further research to understand the long-term effects and safety of 4D-printed implants and tissues [38].
In general, the possible response to certain stimuli has to be achieved in bioprinted constructs; otherwise, they would not fulfill their purpose. An example would be the innervation and response of muscle tissue to stimuli.
If in the future, whole organs can be printed, the implantation and transplantation guidelines might need to be updated and be adjusted to the new field of bioprinting. The ethical aspects for the translation of in vitro studies and animal in vivo studies in human application need special consideration. International guidelines must be drawn up to regulate the use of bioprinting in medicine [39].
References
Zhou G et al (2018) In vitro regeneration of patient-specific ear-shaped cartilage and its first clinical application for auricular reconstruction. EBioMedicine 28:287–302
Hull, CW, Apparatus for production of three-dimensional objects by stereolithography. US Pat 4575330., (1986)
Dhariwala B, Hunt E, Boland T (2004) Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng 10(9-10):1316–1322
Levato R et al (2020) From shape to function: the next step in bioprinting. Adv Mater 32(12):e1906423
Guba M et al (2022) Tissue engineered skin products in research and therapeutic applications. Orv Hetil 163(10):375–385
Groll J et al (2016) Biofabrication: reappraising the definition of an evolving field. Biofabrication 8(1):013001
Gershlak JR, Ott HC (2020) Bioprinting organs-progress toward a moonshot idea. Transplantation 104(7):1310–1311
Satpathy A et al (2018) Developments with 3D bioprinting for novel drug discovery. Expert Opin Drug Discovery 13(12):1115–1129
O’Connell RL et al (2015) Review of three-dimensional (3D) surface imaging for oncoplastic, reconstructive and aesthetic breast surgery. Breast 24(4):331–342
Mu X, Zhang J, Jiang Y (2021) 3D printing in breast reconstruction: from bench to bed. Front Surg 8:641370
Moroni S, Casettari L, Lamprou DA (2022) 3D and 4D printing in the fight against breast cancer. Biosensors (Basel) 12(8):568
Rogers-Vizena CR et al (2017) The current role of three-dimensional printing in plastic surgery. Plast Reconstr Surg 139(3):811e–812e
Busch LF, Alawi SA (2020) Evaluation of patients’ preferences for skin grafting in plastic-surgical defect coverage. World J Plast Surg 9(3):259–266
Werner D, Alawi SA (2019) Four extremity amputation and bionic prosthesis supply after disseminated intravascular coagulation: a follow-up on functionality and quality of life after bionic prosthesis supply. World J Plast Surg 8(2):146–162
Alawi SA et al (2018) Quality of life and reconstructive surgery efforts in severe hand injuries. Innov Surg Sci 3(2):147–156
Roshangar L et al (2021) Using 3D-bioprinting scaffold loaded with adipose-derived stem cells to burns wound healing. J Tissue Eng Regen Med 15(6):546–555
Tamay DG et al (2019) 3D and 4D printing of polymers for tissue engineering applications. Front Bioeng Biotechnol 7:164
Zadpoor AA, Malda J (2017) Additive manufacturing of biomaterials, tissues, and organs. Ann Biomed Eng 45(1):1–11
Dey M, Ozbolat IT (2020) 3D bioprinting of cells, tissues and organs. Sci Rep 10(1):14023
Cubo N et al (2016) 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication 9(1):015006
Varkey M et al (2019) Skin bioprinting: the future of burn wound reconstruction? Burns Trauma 7:4
Yanez M et al (2015) In vivo assessment of printed microvasculature in a bilayer skin graft to treat full-thickness wounds. Tissue Eng Part A 21(1-2):224–233
Abaci HE et al (2018) Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat Commun 9(1):5301
Song P, Pu LLQ (2018) The soleus muscle flap: an overview of its clinical applications for lower extremity reconstruction. Ann Plast Surg 81(6S Suppl 1):S109–S116
Matschke J et al (2019) AV loop free flap: an interdisciplinary approach for perineal and sacral defect reconstruction after radical oncological exenteration and radiation in a colorectal cancer patient. World J Surg Oncol 17(1):154
Werner D, Alawi SA (2022) Correction to: Hand Bionic Score: a clinical follow-up study of severe hand injuries and development of a recommendation score to supply bionic prosthesis. Eur J Plast Surg 45(1):211
Apelgren P et al (2018) Skin grafting on 3D bioprinted cartilage constructs in vivo. Plast Reconstr Surg Glob Open 6(9):e1930
Kang HW et al (2016) A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34(3):312–319
Shim JH et al (2016) Three-dimensional bioprinting of multilayered constructs containing human mesenchymal stromal cells for osteochondral tissue regeneration in the rabbit knee joint. Biofabrication 8(1):014102
Mancini IAD et al (2020) A composite hydrogel-3D printed thermoplast osteochondral anchor as example for a zonal approach to cartilage repair: in vivo performance in a long-term equine model. Biofabrication 12(3):035028
Yan Y et al (2019) Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 190-191:97–110
Wang L et al (2018) Development of a centrally vascularized tissue engineering bone graft with the unique core-shell composite structure for large femoral bone defect treatment. Biomaterials 175:44–60
Pati F et al (2015) Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 62:164–175
Di Bella C et al (2015) 3D bioprinting of cartilage for orthopedic surgeons: reading between the lines. Front Surg 2:39
Datta P, Ayan B, Ozbolat IT (2017) Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater 51:1–20
Kim JH et al (2018) 3D bioprinted human skeletal muscle constructs for muscle function restoration. Sci Rep 8(1):12307
Li YC et al (2016) 4D bioprinting: the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 9(1):012001
Ashammakhi N et al (2018) Advances and future perspectives in 4D bioprinting. Biotechnol J 13(12):e1800148
Gilbert F et al (2018) Print me an organ? Ethical and regulatory issues emerging from 3D bioprinting in medicine. Sci Eng Ethics 24(1):73–91
Acknowledgements
We would like to thank Mr. Raphael William Brooks, M.D., and Mr. Michele Rudari, M.D., for proofreading and linguistic revision of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This is a review article a registration with the local ethics committee was not necessary.
Competing interests
Seyed Arash Alawi, Jan Matschke, David Muallah, Michael Gelinksy, and Adrian Dragu declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alawi, S.A., Matschke, J., Muallah, D. et al. 3D bioprinting in plastic and reconstructive surgery: current concepts, progress, and clinical application. Eur J Plast Surg 46, 833–843 (2023). https://doi.org/10.1007/s00238-023-02108-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-023-02108-7