Abstract
Early detection of vascular compromise after autologous breast reconstruction is crucial to enable timely re-exploration for flap salvage. Several studies proposed non-invasive tissue oximetry for early identification of ischemia of deep inferior epigastric perforator (DIEP) flaps. The present study aimed to explore the utility of non-invasive tissue oximetry following DIEP flap surgery using a personalized oxygenation threshold.
Methods
Patients undergoing immediate/delayed DIEP flap surgery were included in this prospective observational study. DIEP flap tissue oxygenation (StO2) was monitored continuously using near-infrared spectroscopy. A baseline measurement was performed by positioning one sensor at the marked position of the major inferior epigastric perforator on the abdomen. A new sensor was positioned postoperatively on the transplanted tissue. In unilateral procedures, postoperative StO2 values of the native breast were also obtained. Measurements were continued for 24 h.
Results
Thirty patients (42 flaps) were included. Fourteen patients (46.7%) had an uncomplicated postoperative course. A minor complication was observed in thirteen patients; in five patients, at least one major complication occurred, requiring re-exploration. Median StO2 readings were significantly lower in patients with major complications compared to uncomplicated cases. In fourteen unilateral DIEP flap procedures, StO2 values of the native breast were similar to the preoperative baseline measurement (92%; p = 0.452).
Conclusions
Non-invasive tissue oximetry following DIEP flap surgery could aid in early detection of vascular compromise. StO2 values of the native breast and abdominal wall preoperatively can be used interchangeably and can serve as personalized reference value.
Level of evidence: Level IV, diagnostic / prognostic study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advances in microsurgical techniques contributed to deep inferior epigastric perforator (DIEP) flap becoming the preferred choice of postmastectomy autologous breast reconstruction [1, 2]. Current literature shows overall success rates of free flap transfer up to 98% [3,4,5]. Nevertheless, postoperative (partial) flap compromise, for which immediate re-exploration is required to minimize the risk of flap loss, still occurs in 5–25% of the procedures [3, 6]. The time between the onset of malperfusion and surgical intervention is directly related to the salvage rate, and if re-exploration is not performed within 12 h, ischemic tissue damage is likely to be irreversible [6,7,8]. Total or partial loss of a DIEP flap has a major negative impact on the physical and psychological well-being of the patient [5]. Therefore, early detection of vascular compromise, enabling timely re-exploration is crucial in preventing complications contributing to flap failure.
In order to prevent ischemic events following free tissue transfer, real-time and objective monitoring of flap perfusion is crucial. The ideal monitoring technique should be safe for the patient, rapidly responsive, accurate, reliable, applicable to all types of flaps, easy to use, and cost-effective [9, 10]. To date, there is no single device available meeting all previously mentioned criteria [10,11,12]. Rather subjective and intermittently performed methods, such as clinical observation of skin color and flow measurements using Doppler ultrasonography remain gold standard for assessment of flap viability [2, 3, 11, 13].
Non-invasive tissue oximetry allows for continuous bedside monitoring of regional tissue oxygen saturation (StO2) [14]. Since its introduction as a monitoring tool for flap viability [4, 15], several studies suggested that tissue oximetry could be used as a reliable diagnostic modality for early detection of tissue hypoxia in free flaps [16, 17]. To date, no consensus has been established on adequate interpretation strategies of StO2 data in the clinical setting [15, 18]. Nevertheless, among the existing studies the criterion described by Keller et al. [17] (StO2 values below the absolute value of 30%, or a decrease of 20% from baseline values for longer than 60 min in duration, are considered predictors for circulatory compromise and are mostly adopted [6, 18, 19]. However, anatomical and physiological factors might influence StO2 values within and across patients, as well as across different types of flaps [19].
The aim of the present study was to confirm the usefulness of non-invasive tissue oximetry in postoperative monitoring of DIEP flaps for autologous breast reconstruction, and to incorporate a personalized tissue oxygen threshold for more accurate interpretation of changes in StO2 values.
Material and methods
This study was performed in line with the principles of the Declaration of Helsinki. Institutional review board approval (METC 16–04-037) was obtained prior to conducting this prospective observational study. An informed consent was obtained, in which patients agreed to the use of their data and publication in a scientific journal.
Study subjects
Female patients undergoing unilateral or bilateral, immediate or delayed DIEP flap breast reconstructive surgery at one university medical center were included.
Non-invasive tissue oxygenation
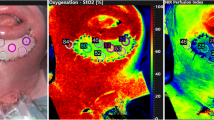
Tissue oxygenation values of the DIEP flap were continuously measured using near-infrared spectroscopy, which is based on the Lambert–Beer law. The proportion of light absorbed by oxyhemoglobin (HbO2) and deoxyhemoglobin (Hb) was determined using five wavelengths within the near-infrared spectrum (700–1100 nm) [15, 20]. Specific absorption spectra of HbO2 and Hb allow for estimation of the concentration of both hemoglobin forms [6, 21]. In the current study, the FORE-SIGHT MC-2030 oximeter (formerly Casmed, Branford, CT, USA, currently Edwards Lifesciences, Irvine, CA, USA) was used to monitor StO2. To incorporate a personalized baseline measurement, a single disposable self-adhesive sensor was positioned on the abdominal area at the position of the major inferior epigastric perforator prior to surgery. Guided by a preoperative computed tomography (CT) and/or a magnetic resonance imaging (MRI) scan and a handheld Doppler ultrasonography device, the anatomical location of the major perforator was assessed from which the baseline StO2 was derived by calculating the mean/median from measurements approximately 5 min in duration. When after preoperative assessment more perforators were found appropriate to use for the reconstruction, an individual baseline measurement was performed for each perforator. Immediately following surgery, a new sensor was placed on the DIEP flap as close to the major flap perforator as possible, near to the reference point for the postoperative Doppler measurement. The surgeon reported which perforator was used during surgery and the corresponding baseline measurement was utilized for analysis. The sensors accompanying the tissue oximeter device cannot be sterilized; hence, it was necessary to use a new sensor for the tissue oxygenation assessment postoperatively. A DIEP flap dimension of at least 50 mm by 30 mm was required to allow proper sensor placement. In case of a unilateral breast reconstruction, postoperative StO2 values of the native breast were obtained as well, with another sensor positioned on the lower site of the breast below the areola. At the time of patient arrival at the recovery room, the sensors were connected to the clinical oximeter to initiate the postoperative continuous measurement for 24 h, see Fig. 1. This study had a prospective observational design. Therefore no decisions regarding possible flap viability were made based on the tissue oximetry values. Clinical observation (i.e., capillary refill, flap color, temperature) and Doppler measurements were performed according to hospital protocol and were leading for decision-making.
Statistical analysis
For the a priori sample size calculation, a difference in StO2 of 10% and a delta StO2 per hour of 10% were considered clinically relevant changes, taking into account the inaccuracy of the clinical oximeter of 4.7%. With alpha set at 0.05, a statistical power (1-β) of 0.95, and σ of 13.75 (maximum – minimum divided by 4, i.e., 99–44/4), the estimated sample size was 28 patients (the maximum and minimum StO2 detectable values are 99 and 44%, respectively). Results were analyzed based on the total number of flaps. Data were divided into three groups based on postoperative complications. No complications, minor complications (e.g., ecchymosis), and major complications (all complications requiring re-exploration). Depending on data distribution, variables were presented as means ± standard deviation (SD) or median [interquartile range] (IQR).
Differences in StO2 values between groups were assessed using the one-way Anova or Kruskal Wallis test. If a significant result was yielded, pairwise comparisons were performed using a Bonferroni correction. In case of a significant result from the Kruskal Wallis test, pairwise comparisons with a manual Bonferroni correction were used. The manual Bonferroni correction was performed by multiplying the derived p-value by the number of pairwise comparisons. To assess differences between preoperative and postoperative StO2 values, a paired samples T-test or Wilcoxon signed rank test was used, depending on data distribution. For comparison of StO2 values between the native breast and the preoperative measurement, the independent samples T-test or Mann–Whitney U-test was used.
For all tests, a two-tailed p-value < 0.05 was considered statistically significant. All analyses were performed using SPSS (version 25.0, SPSS Inc., Chicago, IL, USA) for Windows.
Results
Thirty patients, who underwent DIEP flap breast reconstruction, were included. In four patients who gave their consent, the dimension of the skin-island of the DIEP flap was too small. Patients could therefore not be included in this study. There were no patients excluded for other reasons. The mean age at the time of surgery was 51 ± 13 years (Table 1). Patients had a mean body mass index of 27.5 ± 4.3 kg/m2. Approximately 50% of patients received adjuvant therapy prior to surgery, consisting of radiation therapy, chemotherapy, endocrine therapy, immunotherapy, or a combination of these. Two patients had a history of tobacco smoking.
Fourteen patients (46.7%) underwent unilateral breast reconstruction, while sixteen (53.3%) underwent a bilateral reconstruction. This resulted in a total of 46 DIEP flaps, consisting of 28 immediate (60.9%) and 18 (39.1%) delayed procedures. In four flaps, the skin island was too small to allow proper sensor placement; these cases were excluded from the study. As a result, data of 42 DIEP flaps (in 30 patients) were analyzed. The mean flap weight of all DIEP flaps was 755 ± 235 g (782 ± 199 g and 690 ± 254 g for immediate and delayed DIEP flap procedures respectively; p = 0.768). The median ischemic time period was 42 [34–51] min, as shown in Table 2. In addition, in the unilateral DIEP flap reconstruction, postoperative StO2 values of the native breast were also obtained. The median StO2 reading from the native breast was 92 [92–93]%. This value was not significantly different compared to the preoperative baseline measurement on the abdomen (92 [91–93]%; p = 0.452).
In fourteen patients (46.7% of patients, accounting for 19 flaps, 45% of all flaps), an uncomplicated postoperative course was observed. In these procedures, postoperative StO2 readings showed to be relatively stable (with maximum fluctuations of 3%) throughout the entire measurement and did not differ significantly from preoperative StO2 values (median values of 92 [89–92] and 93 [91–93], respectively). Figure 2 depicts an example of StO2 readings in case of an uncomplicated recovery.
One of the patients with an uncomplicated postoperative course showed an atypical pattern of StO2 values. In contrast to the other uncomplicated cases, the ischemic time period during this particular procedure was relatively long, 130 min, as compared to the median of 42 min. Tissue oximetry readings showed a two-step recovery phase of each approximately 2 h in duration (Fig. 3). After 6 h, postoperative StO2 values were similar to baseline values.
A minor complication (e.g., ecchymosis) was observed in thirteen patients (43.3% of patients, accounting for seventeen flaps, 41% of total flaps). In these cases, postoperative median StO2 values were not significantly different as in the uncomplicated procedures. Patients with either no or minor complications were discharged from the hospital after a median of 5 [4-5] days of hospital stay.
In five patients (16.7%), at least one major complication occurred in which re-exploration was required. A unilateral DIEP flap reconstruction was performed in four patients. A bilateral reconstruction was performed in one patient; in both DIEP flaps, a complication occurred. In total, a major complication was noticed in five DIEP flap reconstructions (12% of all flaps), and one re-exploration was due to a donor site complication. Patient 1 was diagnosed with fat necrosis. This was debrided during surgery. Patient 2 was diagnosed with an arterial inflow problem. During the re-operation, the arterial anastomosis was kinked, which was corrected by repositioning of the pedicle. In patient 3, evacuation of a haematoma was performed: during the re-exploration, a minor arterial bleeding of the pectoralis muscle was found. In patient 4, a bilateral reconstruction was performed. On the left side, the patient received a large flap (1200 g). The used perforator was insufficient to create an optimal perfusion for this flap, leading to partial loss of the caudal side of the flap. Therefore, 100 g of the flap was ultimately removed. In the right DIEP flap, venous congestion occurred. During re-exploration, the venous anastomosis appeared to be kinked, which was corrected by repositioning. Patient 5 did not have any complications on the DIEP flap, but developed a massive acute bleeding in the donor site (1500 mL blood loss), which had a major impact on the StO2-values and on the outcome of the clinical assessment of the DIEP flap. The median StO2 readings were significantly lower in patients with major complications compared to patients with an uncomplicated postoperative course: 80 [79–84]% vs. 92 [89–92]% (p < 0.001). Furthermore, patients with a complicated course (minor and/or major) showed a significantly higher body mass index (BMI) (30.0 ± 3.7 kg/m2 and 32.1 ± 4.9 kg/m2, respectively) than patients with an uncomplicated course (24.9 ± 3.4 kg/m2; p < 0.001). Additionally, the flap weight of DIEP flap procedures which were followed by major complications (1040 ± 173 g) was significantly higher compared to patients with no complications (608.0 ± 161.6 g; p < 0.001) or minor complications (829.5 ± 217.5 g; p = 0.049). As shown in Table 3, flap weight was also significantly different between patients without complications (608.0 ± 161.6 g; p < 0.001) and minor complications (829.5 ± 217.5 g; p = 0.003).
Postoperative StO2 values did not significantly differ from preoperative (baseline) values (p = 0.069 and p = 0.104 in patients without complications or minor complications, respectively). In patients with major complications, postoperative StO2 readings were significantly lower compared to preoperative values (p = 0.013).
In all cases, no StO2 values below the absolute value of 50% were observed. In cases in which surgical flap re-exploration was required, a decrease of 20% or more from baseline values for more than 60 min was observed in two out of six cases (33.3% of cases). The most substantial decrease observed in this study was a relative 37% decrease from baseline for more than 4 h in duration, which occurred in one particular patient (Fig. 4). During early postoperative recovery, it appeared that blood flow towards the caudal side of the left flap was impeded, for which revisional surgery was required to allow tissue salvage. On the fifth postoperative day, 100 g of the total flap weight had to be surgically removed due to compromised tissue perfusion.
Discussion
Early detection of vascular compromise in autologous breast reconstruction is essential to pursue successful flap salvage. This prospective observational study illustrated that non-invasive tissue oximetry can successfully identify patients at risk of major clinical complications following DIEP flap reconstruction: visual aspects of tissue oximetry readings in complicated cases were significantly different from uncomplicated procedures. In addition, a personalized tissue oxygen threshold was incorporated.
In almost all patients with uneventful recovery, postoperative StO2 values of the DIEP flap immediately matched the preoperative baseline values. Five patients suffered from a major complication, requiring surgical re-exploration for salvage of the free flap. Tissue oximetry readings in these cases showed a significantly lower median StO2 (80 [79–84]%) compared to preoperative baseline values (92 [89–92]%; p = 0.002). This is in line with previous studies by Vranken et al., Keller et al., and Repez et al. [6, 17, 22] which suggested that postoperative StO2 readings could identify vascular compromise in an early postoperative phase.
While general consensus on the required duration of postoperative monitoring is still lacking, the current study (with a 24-h continuous postoperative monitoring window) was able to detect major complications in a predefined timeframe. Previous studies used monitoring windows with varying durations, mostly between 24 and 48 h postoperatively [1, 17, 22]. A recent study by Carruthers et al. showed that microvascular issues become visible during clinical evaluation in the initial 23 h following reconstructive surgery [23], suggesting that a 24-h time window should be adequate. These results in combination with the results of the current study suggest that continuous postoperative monitoring of the DIEP flap with the use of tissue oximetry for longer than 24 h would not provide additional important information and therefore a timeframe of 24 h of continuous postoperative tissue oximetry monitoring could be justified. Nevertheless, clinical observation (i.e., capillary refill, flap color, temperature) and Doppler measurements should be continued according to hospital protocol.
A substantial number of studies consider high BMI and high flap weight to be risk factors for complications following breast reconstructive surgery [24,25,26,27,28]. In this study, the BMI values of patients without complications were significantly lower compared to patients with minor or major complications (32.1 ± 4.9 kg/m2). Weight of the DIEP flap was also significantly lower in patients without complications compared to patients with minor or major complications. These findings are in line with the literature.
One way to effectively assess flap perfusion in the early postoperative phase would be to apply a baseline value to objectify relevant changes in tissue StO2 in real time [17]. The thresholds described previously by Keller et al., StO2 below 30%, or a decrease of 20% from baseline values for longer than 60 min in duration, are considered predictive of circulatory compromise and used in several clinical centers [6, 17]. Various studies have questioned the thresholds as proposed by Keller and colleagues [13, 19, 29]; they suggested adaptions of the thresholds to an absolute value of 40%, or a decrease of 15% from baseline for longer than 60 min [13, 19]. In line with the studies of Steele et al. and Hölze et al. [13, 29], in the current study, no absolute StO2 values below 55% were observed. Furthermore, we found a relative decrease of 20% for at least 60 min was only observed in 33% of cases in which a re-exploration (6 out of 42 flaps) was needed.
There are several types of commercially available oximeters, all use different types of sensors and different wavelengths of near-infrared light. In addition, the assumed ratio between arterial saturation and venous saturation in the equation to calculate the reference values varies between devices [30]. In a study of Hyttel-Sorensen and colleagues, a comparison of three different oximeters showed different absolute measurement values for all the devices [31]. In the study of Hölzle (O2C (Oxygen 2 see, LEA CO., Gießen, Germany) as well in the study of Akita (TOS-OR (Fujita Medical Instruments Co., Ltd., Tokyo, Japan), a different type of oximeter was used compared to the study of Keller (ViOptix T.Ox Tissue Oximeter (ViOptix Inc., Fremont, CA, USA) [18, 19, 29]. This could also explain the variations in StO2 patterns across uncomplicated cases between this study and the study by Vranken et al.; both studies included patients in the same medical center. While Vranken et al. used the INVOS™ 5100C Cerebral/Somatic Oximeter (Medtronic, Minneapolis, MN, USA), in the current study, the FORE-SIGHT MC-2030 oximeter was used [22]. Direct comparison of StO2 values of different oximeters is therefore not recommended.
The StO2 values observed in patients without complications and patients with minor complications were not significantly different, and in neither group values below 88% were detected. In patients with at least one major complication, on the other hand, median StO2 values were significantly lower (80 [79–84]%). Postoperative values of this group were also significantly lower compared to the preoperative baseline values (93 [86–93]%). Due to the relatively small patient population and occurrence of complications in this current study, objective determination of fixed cut-off values below which surgical re-exploration is indicated was not feasible. Furthermore, as described in a study of Bickler et al., interindividual differences in tissue oxygenation values with the present oximetry technology introduce a major challenge in determining a threshold for regional tissue desaturation [30]. Even though the improvement in monitoring techniques contributes to the ability to detect vascular compromise before clinical evidence for circulatory failure becomes apparent [17, 31, 32]. These findings illustrate that readings from different devices cannot be directly compared, indicating that device-specific, sensor-specific, and measurement site-specific cut-off values should be considered when determining tissue desaturation thresholds requiring immediate intervention to prevent adverse clinical effects [31, 33, 34].
Nevertheless, the current study included fourteen unilateral DIEP flap cases, incorporating the native breast as a personalized reference measurement. StO2 readings of the native breast showed similar values compared to preoperative baseline readings from the abdomen. This suggests that for unilateral as well as bilateral DIEP flap reconstructions, a personalized reference could be obtained. In case of a unilateral procedure, this would mean that if no baseline measurement could be performed preoperatively, postoperative monitoring of the DIEP flap with use of tissue oximetry could still be performed using a baseline from the contralateral breast. However, in ahead of implementing this reference, its reliability needs further analysis.
Evidence regarding the use of a personalized reference for postoperative DIEP flap monitoring using tissue oximetry remains limited. The StO2 values of the preoperative measurement could be biased due to subjectivity while applying Doppler ultrasonography in order to detect to location of the major perforator in the flap still in situ. However, in most cases, a preoperative CT and/or MRI was conducted to localize the major perforator. Accordingly, preoperative StO2 values could still be affected due to interference of other vessels. A more reliable method would be to measure the StO2 values of the flap during surgery, when the flap is only vascularized by the dissected pedicle from the major inferior epigastric perforator. Nevertheless, there are no sterile sensors available in Europe to measure tissue oxygenation intraoperatively; therefore, a more reliable assessment is not achievable. Another option to assess tissue oxygenation intraoperatively would be the use of a hyperspectral imaging camera [35].
Nonetheless, the sample size in this study was limited. Thirty patients (42 flaps) were included, but in only six cases, a major complication occurred. This could be an explanation that in this study no false-positive cases were found. Another limitation was that no comparison could be made between an accurate and timely proper physical exam, Doppler measurements, and the NIRS-measurement in the early postoperative period. Therefore, no hard conclusions could be made if tissue oximetry would be superior to a physical exam or Doppler measurements. For that reason, a randomized control trial with a larger sample size and intervention and control group would be needed.
In conclusion, non-invasive tissue oximetry following DIEP flap breast reconstruction can enable early identification of patients at risk of major clinical complications in the first 24 h after surgery. Preoperative tissue oximetry values at the DIEP flap donor site and postoperative values at the native breast can be used interchangeably as a personalized reference threshold. Device-specific and measurement site-specific cut-off values should be identified instead of a general threshold for dangerous lower level for StO2.
Data availability
Available on request.
Code availability
Not applicable.
References
Salgarello M, Pagliara D, Rossi M, Visconti G, Barone-Adesi L (2018) Postoperative monitoring of free DIEP flap in breast reconstruction with near-infrared spectroscopy: variables affecting the regional oxygen saturation. J Reconstr Microsur 34(06):383–388
Whitaker IS, Pratt GF, Rozen WM, Cairns SA, Barrett MD et al (2012) Near infrared spectroscopy for monitoring flap viability following breast reconstruction. J Reconstr Microsurg 28(3):149–154
Pruimboom T, van Kuijk SMJ, Qiu SS et al (2020) Optimizing indocyanine green fluorescence angiography in reconstructive flap surgery: a systematic review and ex vivi experiments. Surg innovat 27(1):103–119
Irwin MS, Thorniley MS, Doré CJ, Green CJ (1995) Near infra-red spectroscopy: a non-invasive monitor of perfusion and oxygenation within the microcirculation of limbs and flaps. Br J Plast Surg 48(1):14–22
Pelletier A, Tseng C, Agarwal S, Park J, Song D (2011) Cost analysis of near-infrared spectroscopy tissue oximetry for monitoring autologous free tissue breast reconstruction. J Reconstr Microsurg 27(8):487–494
Repez A, Oroszy D, Arnez ZM (2008) Continuous postoperative monitoring of cutaneous free flaps using near infrared spectroscopy. J Plast Reconstr Aesthet Surg 61(1):71–77
Siemionow M, Arslan E (2004) Ischemia/reperfuion injury: a review in relation to free tissue transfers. Microsurgery 24(6):468–475
Smits JM, Acosta R, Zeebregts CJ et al (2007) Early reintervention of compromised free flaps improves succes rate. Microsurgery 27:612–616
Creech B, Miller S (1975) Evaluation in circulation in skin flaps. In: Grabb WC, Meyers MB (eds) Skin flaps. Little Brown, Boston, pp 21–38
Smit JM, Zeebregts CJ, Acosta R, Werker PM (2010) Advancements in free flap monitoring in the last decade: a critical review. Plast Reconstr Surg 125:177–185
Colwell AS, Craft RO (2011) Near-infrared spectroscopy in autologous breast reconstruction. Clin Plast Surg 38(2):301–302
Cervenka B, Bewley AF (2015) Free flap monitoring: a review of the recent literature. Curr Opin Otolaryngol Head Neck Surg 23:393–398
Steele MH (2011) Three-year experience using near infrared spectroscopy tissue oximetry monitoring of free tissue transfers. Ann Plast Surg 66(5):540–545
Jobsis FF (1977) Non-invasive infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. AAAS 198:1264–1267
Thorniley MS, Sinclair JS, Barnett NJ, Shurey CB, Green CJ (1998) The use of near-infrared spectroscopy for assessing flap viability during reconstructive surgery. Br J Plast Surg 51:218–226
Kagaya Y, Miyamoto S (2018) A systematic review of near-infrared spectroscopy in flap monitoring: current basic and clinical evidence and prospects. JPRAS 71(2):246–257
Keller A (2009) A new diagnostic algorithm for early prediction of vascular compromise in 208 microsurgical flaps using tissue oxygen saturation measurements. Ann Plast Surg 62(5):538–543
Berthelot M, Ashcroft J, Boshier P et al (2019) Use of near-infrared spectroscopy and implantable Doppler for postoperative monitoring of free tissue transfer for breast reconstruction: a systematic review and meta-analysis. Plast Reconstr Surg Glob Open 7:e2437
Akita S, Mitsukawa N, Tokumoto H et al (2017) Regioal oxygen saturation index: a novel criterion for free flap assessment using tissue oximetry. Plast Reconstr Sur 139:1213e–1214e
Murkin JM, Arango M (2009) Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 103(Suppl. 1):i3–i13
Lohman RF, Langevin CJ, Bozkurt M, Kundu N, Djohan R (2013) A prospective analysis of free flap monitoring techniques: physical examination, external Doppler, implantable Doppler, and tissue oximetry. J Reconstr Microsurg 29:51–55
Vranken NPA, Weerwind PW, van Onna MA, Bouman EAC, van der Hulst RRWJ (2017) Non-invasive tissue oximetry following unilateral DIEP-flap reconstruction: a pilot evaluation. JPRAS Open 12(Supplement C):59–65
Carruthers KH, Tiwari P, Yoshida S (2019) Inpatient flap monitoring after deep inferior epogastric artery perforator flap breast reconstruction: how long is long enough? J reconstr Microsurg 35:682–687
Garvey PB (2012) The advantages of free abdominal-based flaps over implants for breast reconstruction in obese patients. Plast Reconstr Surg 130(5):991–1000
Vyas RM, Dickinson BP, Fastekjian JH, Watson JP, DaLio AL et al (2008) Risk factors for abdominal donor-site morbidity in free flap breast reconstruction. Plast Reconstr Surg 121(5):1519–1526
Andree C, Langer S, Seidenstuecker K, Richrath P, Behrendt P et al (2013) A single center prospective study of bilateral breast reconstruction with free abdominal flaps: a critical analyses of 144 patients. Med Sci Monit 19:467–474
Massenburg BB, Sanati-Mehrizy P, Ingargiola MJ, Hernandez Rosa J, Taub PJ (2015) Flap failure and wound complications in autologous breast reconstruction: a national perspective. Aesth Plast Surg 39:902–909
Beugels J, Bod L, van Kuijk SMJ, Qiu SS, Tuinder SMH, Heuts EM et al (2018) Complications following immediate compared to delayed deep inferior epigastric artery perforator flap breast reconstructions. Breast Cancer Res Treat 169:349–357
Hölzle F, Loeffelbein DJ, Nolte D, Wolff KD (2006) Free flap monitoring using simultaneous non-invasive laser Doppler flowmetry and tissue spectrophotometry. JCMS 34:25–33
Bickler PE, Feiner JR, Rollins MD (2013) Factors affecting the performance of 5 cerebral oximeters during hypoxia in healthy volunteers. Anesth Analg 117(4):813–823
Hyttel-Sorensen S, Hessel TW, Greisen G (2014) Peripheral tissue oximetry: comparing three commercial near-infrared spectroscopy oximeters on the forearm. J Clin Monit Comput 28:149–155
Keller A (2007) Non-invasive tissue oximetry for flap monitoring: an initial study. J reconstr Microsurg 23:189–197
Keller A (2011) Non-invasive tissue oximetry. Clin Plast Surg 38(2):313–324
Bevan PJ (2015) Should cerebral near-infrared spectroscopy be standard of care in adult cardiac surgery? Heart Lung Circ 24:544–550
Lu G, Fei B (2014) Medical hypersprectral imaging: a review. J Biomed Opt 19(1):010901
Acknowledgements
The authors acknowledge Ms. VGH Rutjens and Ms. SFC Schins for their practical support in inclusion of patients and conducting the measurements.
Funding
This study was in part funded thru an investigator-initiated research grant from Casmed (Branford, CT, USA) that also provided technical support for specific data extraction.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by the first and second author. The first draft of the manuscript was written by the first author and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript,
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Institutional review board approval (METC 16–04-037) was obtained prior to conducting this prospective study.
Informed consent
An informed consent was obtained, in which patients agreed to the use of their data and publication in a scientific journal.
Patient consent
The authors affirm that human research participants provided informed consent for sharing their data and publication of the images in Fig. 1a and 1b.
Conflict of interest
Anouk A.M.A. Lindelauf, Nousjka P.A. Vranken, Rutger M. Schols, Esther A.C. Bouman, Patrick W. Weerwind, and René R.W.J. van der Hulst declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lindelauf, A.A.M.A., Vranken, N.P.A., Schols, R.M. et al. Exploring personalized postoperative non-invasive tissue oximetry in DIEP flap breast reconstruction. Eur J Plast Surg 45, 267–275 (2022). https://doi.org/10.1007/s00238-021-01873-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-021-01873-7