Abstract
Background
The first description of local fascio-cutaneous flaps used for the coverage of soft tissue defects of the limbs originates from the 1980s. Over the years, this technique has advanced, and in the meantime, a sub-group of flaps named perforator-based propeller flaps has gained increased attention. In our study, we aimed to demonstrate our experience of operating surgeries with perforator-based propeller flaps and to compare this technique with other flap techniques, which are to reconstruct tissue defects of the knee, lower leg, and foot.
Methods
A systematic retrospective search for flap procedures for defect reconstructions in the knee, lower leg, and foot from our database was performed. All data between January 2010 and August 2018 were considered. We identified 56 procedures performed on 14 female and 42 male patients with the mean age of 54.13 years. Reconstruction procedures consisted of 34 free flaps, 14 perforator-based propeller flaps, and eight other perforator-based flaps. Compared to free flaps, the perforator-based propeller flaps had shorter surgery duration by 46.6% (p < 0.0001) and the complication rate in the cases of perforator-based propeller flaps was reduced by 31.14% (p = 0.0315). Furthermore, the operations carried out with perforator-based propeller flaps resulted in a significantly lower rate of revisions by 36.03% (p = 0.0204), compared to those with free flaps. The majority of the donor sites of free flaps were self-closing with the direct suture (p = 0.004).
Conclusions
Based on our findings, we can propose the applicability of perforator-based flaps in treating defects of the knee, lower leg, and foot. With a correct indication, perforator-based propeller flap represents a promising alternative to free flaps, with its significantly shorter surgery duration, lower complications rate, and lower revision rate. However, both techniques of the free flap transfer and the transfer of local pedicle-based flap possess their advantages and disadvantages. Therefore, it is hard to define which microsurgical technique is exclusive in treating lower leg defects.
Level of evidence: Level IV, therapeutic study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first description of the local fascio-cutaneous flaps used for the coverage of soft tissue defects of the limbs was from Ponten in 1981 [1]. Thanks to their anatomical studies, Palmer and Taylor defined static vascular territories of each main vessel and their perforators as angiosomes [2]. Moreover, Saint-Cyr et al. defined the vascular territory supplied by a single perforator as perforasome [3]. The term “perforator-based flap” was introduced by Kroll and Rosenfield, and in 1991, the term “propeller flap” was proposed by Hyakusoku, who first described an adipo-cutaneous flap, which could be rotated only by 90° on the pedicle as a pivot point. The limited rotation of the skin island was due to the location of random pedicle completely embedded in adipose subcutaneous tissue. The longitudinally shaped skin island, with its length exceeding the width, consisted of two paddles, one longer and one shorter, similar to the blades of a plane propeller [4].
Also, Hallock introduced further improvement by skeletonizing the central pedicle, which was then set free from fat tissue and fascial or septal adhesions. This maneuver allowed more rotational movement of the skin island up to 180°, without buckling the nourishing perforator. While the longer paddle of the flap covered the defect, the shorter was placed over the donor site and contributed to its direct closure. This technique is still valid nowadays [5].
According to Advisory Panel of the First Tokyo Meeting on Perforator and Propeller Flaps in June 2009, a propeller flap can be described as an “island flap that reaches the recipient site through an axial rotation". Island flaps moved in other ways, e.g., by the advancement, or incomplete islanded flaps, are excluded from the aforementioned definition [6].
Similar to the proposals of the “Gent Consensus on Perforator Flap Terminology” in 2001, there are some demands on the classification of propeller flaps. A correct classification should include the type of nourishing pedicle (subcutaneous-pedicled, perforator-pedicled, and supercharged propeller flap), the degree of rotation, and the artery of origin of the perforator. Thus, perforator-based propeller flaps should be identified based on the skin’s source of the nutrient vessel, not on the underlying muscle [7, 8].
Since then, many new findings were published, contributing to improvements in surgical techniques, identification of risk factors, and prevention of postoperative complications. With our retrospective evaluation, we aimed to present our experience of performing the perforator-based flaps and comparing it with other flap techniques in reconstructing tissue defects of the lower leg.
In our opinion, parameters such as surgery duration, duration of the postoperative on-ward-stay, complication rate, and revision surgery rate are essential, concerning both medical and economical aspects of treatments.
Materials and methods
For the retrospective evaluation, we extracted the data from the electronic, photographic, and paper-based documentation. We included data collected between 2010 and 2018.
Only patients, who had defects of the knee, lower leg, and foot and were treated with free flaps (ff), perforator-based propeller flaps (ppf), or other perforator-based flaps (opf), were included in our investigation. We coined a term other perforator-based flaps (opf) to incorporate further different types of flaps such as local freestyle perforator-pedicled advancement or transposition flaps, and local random pattern advancement or transposition flaps into a collective term. The included parameters for our evaluations were gender, age, diagnose, pre-operative diagnostics, surgical procedure, duration of surgery, arch of propeller rotation, duration of postoperative hospitalization, complications, revision surgeries, donor-site closure, and recipient vessel or angiosome. Statistical analyses for surgery duration, duration of postoperative on-ward-stay, complication rate, and revisions rate were conducted using GraphPad Prism 5.04 (GraphPad Software, San Diego, CA, USA). One-way analysis of variance (ANOVA, p < 0.05) was applied to reveal the differences between the treatments. The differences between individual means were estimated using Tukey post hoc test for multiple comparisons with a significance level of 0.05 (95% confidence interval) (***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05). Data in the figures were displayed with means and standard errors of the means.
Preparation of surgery
In preparation of the surgery, we performed a conventional angiography unless the surgery was an urgent case, or some other diagnostic tools, such as contrast-enhanced MRI or CT, were already acquired and available. The day before surgery, we ensured the availability of a proper perforator by using a hand-held Doppler on the ward.
Surgical procedure
We started surgery by locating the perforator with a hand-held Doppler and marked its location with a pen. Only the supine position was employed on the operating table. A tourniquet was placed on the patient’s thighs, but there was no necessity to inflate it in any of our operations. After the debridement of the defect, the precise planning of flap position, shape, and size was possible. Previously marked area of the perforator created a pivot point of the propeller.
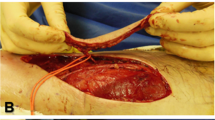
We made an incision along with the previous marks close to the supposed perforator position until the perforator was visible. Once we established its location, the dissection of the pedicle started. This surgical step took the longest time and was technically the most challenging. We always attempted to free the pedicle until the main vessel was reached. Thus, we secured the buckling-free rotation of the pedicle. However, we did not separate the pedicle artery from veins to avoid accidental vessel damage (Fig. 1). Then, we completed the rest of the circumcision around the planned flap (Fig. 2).
At this point, after the dissection beneath the fascia, the rotation of the propeller was completed, and it was placed onto the defect (Figs. 3 and 4). Fitting of the flap precisely into the defect site sometimes required minor corrections such as excisions of small areas of the flap. For drainage, we placed 1–4 easy-flow drains.
Donor site closure was made with the shorter flap paddle and by a skin graft or suturing of the rest of the wound (Figs. 5 and 6). We completely sealed all of the cases with ppf with vacuum-assisted dressings for five to seven days. Vacuum-assisted dressing covered both the flap and donor site. Postoperatively, the patient was not allowed to mobilize for five days.
We operated the surgeries only under a 4.3-fold loop magnification. This magnification was appropriate and more importantly, in our opinion, in contrast to the use of the surgical microscope, the use of surgical loops allowed to observe the structures from various angles effortlessly.
Our technique of raising other flaps, including the ff, did not differ from the methods described in other relevant literature. All of the opf and ff were covered intra-operatively with vacuum-assisted dressing in the same way as the ppf were. Donor sites of ff were covered with conventional sterile dressings without the vacuum assistance.
Results
Diagnostics
The results of the pre-operative diagnostics will be described in a separate publication.
Patients
A total of 56 patients (14 female and 42 male patients) with the mean age of 54.13 years (range: 2–82 years) were included. The patients were categorized into three groups: patients who underwent reconstructive surgeries with ff, ppf, and opf. The ff group consisted of 34 patients (7 female and 27 male patients) with the mean age of 55.18 years (range: 23–82 years). The ppf group had 14 patients (4 female and 10 male patients) with the mean age of 52.38 years (range: 17–72 years). Lastly, the opf group had 8 patients (3 female and 5 male patients) with the mean age of 52.75 years (range: 2–78 years).
Etiology
Indications for surgeries were provided due to five main reasons: trauma in 20 cases (35.71%), delayed wound healing in 14 cases (25%), chronic infections in 13 cases (23.21%), chronic ulcers in five cases (8.93%), and oncological diseases in four cases (7.14%). Four of 20 traumatized patients (20%) had additional severe injuries. Thirty-two of all 56 patients (57.14%) showed additional diagnoses; yet, in one case (1.78%), no data concerning this aspect was available. Most common co-morbidities were arterial hypertonia in 17 cases (30.36%), peripheral artery disease in 16 cases (28.57%), diabetes in 12 cases (21.43%), and hypercholesterolemia in five cases (8.93%). Furthermore, there were some isolated cases of paroxysmal atrial fibrillations, morbid adiposity, renal insufficiency, chronic obstructive pulmonary disease, alcohol or cigarette abuse, pulmonary fibrosis, colitis ulcerosa, essential thrombocythopenia, hypothyreosis, osteoporosis, and therapeutic anticoagulation.
Flaps and surgery statistics
Seven types of ff were operated: 15 gracilis muscle flaps, eight rectus abdominis muscle flaps, five latissimus dorsi muscle flaps, three ALT flaps, one VRAM flap, one radialis fasciocutaneous flap, and one free foot sole flap. Three types of arteries were used as recipient vessels in the ff surgeries: anterior tibial artery, posterior tibial artery, and popliteal artery. The most commonly used recipient vessel was the posterior tibial artery, being used in 18 cases (52.94%). Then, the anterior tibial artery was used in 15 cases (44.12%), and the popliteal artery used in only one case (2.94%). For the ppf surgeries, the posterior tibial artery was used as the perforasome in 10 cases (71.43%), and the anterior tibial artery was used and in four cases (28.57%). No ppf surgery used the popliteal artery as perforasome. In the opf surgeries, the posterior tibial artery was used as a perforasome in five cases (62.5%), and the popliteal artery in two cases (25%). Here, the anterior tibial artery was not used as a perforasome.
The average arc of rotation of ppf was 172.14° (range: 120–180°).
The most common type of donor-site closure in the operations with ff was the primary suture, being performed in 32 of 34 cases (94.1%). Only one patient (2.94%) was required to receive a split-thickness skin graft. For the free transplantation of foot sole after oncological amputation of the lower leg, there was no need for donor-site closure. In the group of ppf, the split-thickness skin graft was used in 11 cases (78.57%); of two cases, the split-thickness skin graft was combined with a partial suture (14.28%). Only in three cases, the donor site was self-closing with primary suture (21.43%). After harvesting opf, a split-thickness skin graft was used for five cases (62.5%), in which one split-thickness skin graft was combined with partial suture (12.5%). In three cases of opf (37.5%), the closure was done only with the suturing. In terms of primary closure of the donor site with the suture alone, the differences between ff and ppf or opf were significant (p = 0.004, one-way ANOVA) (Fig. 7).
In all local perforator-based flaps, only 27.27% of donor sites were closed with suture alone and 72.73% with the use of split-thickness skin graft.
The duration for all surgeries was average of 290.61 min (range: 48–545 min). In the group of ff, the average time needed for the operation was 367.85 min (range: 240–545 min), whereas the groups of ppf and opf were 198.64 min (range: 86–373 min) and 123.25 min (range: 48–234 min), respectively. The average surgery duration of ppf compared to that of ff was significantly shorter by 169.21 min (46.0%, p < 0.0001, one-way ANOVA). Also, the average surgery duration of opf was significantly shorter than that of ff by 244.6 min (66.5%, p < 0.0001, one-way ANOVA) (Fig. 8).
Complications
In the 13 cases from the ff operations (38.24%), complications were noted: five total flap loss (14.70%), three minor local flap necrosis (8.82%) and four wound healing delays (11.76%), and one seroma of the donor site (2.94%). In this group, 14 revision surgeries were required (41.17%). In three cases of the ff operations (8.82%), secondary wound healing without surgical intervention was possible. Among the ppf operations, one case with total flap loss (7.14%) was indicated, making the revision surgery necessary (7.14%). In two cases of opf operations, complications were noted (25%): one case with small local necrosis (12.5%) and one case with small wound dehiscence (12.5%). In both cases, secondary debridement was completed (25%).
There was a significant difference in overall complication rates between ff and ppf (p = 0.0315, unpaired t test, two-tailed). However, no significant differences between ff and opf (p = 0.4941, unpaired t test, two-tailed) and between ppf and opf (p = 0.2612, unpaired t test, two-tailed) were detected.
In terms of total flap loss, there were no significant differences between the ff and ppf (p = 0.4884, Mann-Whitney test, two-tailed), ff and opf (p = 0.2656, Mann-Whitney test, two-tailed), and ppf and opf (p = 0.5083, Mann-Whitney test, two-tailed). Furthermore, no significant differences in partial or small necrosis rates between the three groups were found (ff and ppf, p = 0.2675; ff and opf, p = 0.7769; ppf and opf, p = 0.2193; each Mann-Whitney test, two-tailed).
A significant difference in revision surgery rates was noted only between the operations of the ff and ppf (p = 0.0204, unpaired t test, two-tailed). There was no significant difference between the revision operations of the ff and opf (p = 0.4090, unpaired t test, two-tailed), and between those of ppf and opf (p = 0.2612, unpaired t test, two-tailed). In cases of ppf and opf, no complications of donor sites were recognized.
Postoperative care
Patients who underwent ff operations had postoperative in-patient treatment for an average of 31.41 days (range: 6–92 days), which was the longest among other patient groups. Patients who had operations with ppf had an average of 26.64 days (range: 9–105 days) of postoperative in-patient treatment, and those with opf had an average of 16.87 days (range: 6–31 days). Thus, the length of the postoperative care in-patient treatment for ppf patient group was shorter by 4.77 days (15.19%), compared with ff patient group. Moreover, opf patient group had a shorter duration of postoperative treatment than the ff patient group by 14.54 days (46.3%). In general, the duration of postoperative care for all perforator-based flaps was shorter by 8.32 days (26.49%), compared to that of ff. But all of these results concerning the postoperative care were not statistically significant (p = 0.1839, one-way ANOVA) (Fig. 9).
Discussion
Generally, our strategy in reconstructing the lower leg defects is in following the algorithm proposed by Heller and Levin in 2001 [9,10,11]. In our opinion, operations of soft tissue defects in the lower leg are challenging due to the poor availability of surrounding tissue. Concerning the defect size, exposure of bone, bradytroph structures such as tendons, blood vessels, or nerves, an accurate decision is required to choose the proper type of operational procedures. Risk factors for failure or complications cannot be underestimated, and the advantages and disadvantages of propeller flaps and other flap techniques should be considered.
Our most critical decisive criteria for the use of ppf were the availability or good condition of surrounding soft tissue and the presence of adequate perforators in a proper distance of the defect not exceeding the defect diameter.
In the literature, most defects requiring flap coverage are resulted from trauma (55.2%), then are from oncologic resection (14.1%), chronic ulcer (9.4%), postoperative complications (6.1%), osteomyelitis (4.7%), pressure sore (3.8%), burn injuries (2.8%), donor site closure (2.3%), unstable scars (1.4%), and radionecrosis (0.2%) [12].
Moreover, another study suggested that there is no precise standard or reference for the safe limit of defect size as a guideline for the usage of a particular kind of flap [13]. Some findings demonstrate that a single perforator with a diameter of 0.7 mm is able to supply 47 cm2 of a perforator flap [14]. But it is impossible to presumably estimate the surface of flap vascularized by a single perforator [3]. The surface area of larger than 100 cm2 seems to have no significant influence on the complication rate [12]. Yasir et al. found that a large flap territory of up to 190 cm2 raised on a single perforator is feasible because of the axial communications between the perforasomes within a flap [13] (Figs. 10, 11, and 12). However, Lazzeri et al. reported that in some cases, peripheral necrosis could be caused by inadequate communication between perforasomes [15]. According to Panse et al., there is a six-time higher risk of flap necrosis when the flap length exceeds one-third of the limb length; this study attempted to suggest a more objective and universal size criterion for the flaps, rather than focusing on a numerical threshold for maximal, safe surface of the flaps [16]. However, the ppf and freestyle V-Y advancement flaps are claimed as relatively contraindicated in midsize and large defects [17].
Our assessment of defect size was rather subjective. Unfortunately, there is a lack of accurate description of defect size in our database. Therefore, it was not possible to objectively consider this parameter in our evaluations, and this is a potential source of bias in our study.
The location of the perforator is one of the decisive criteria, as described above, for choosing a type of flap. If the local flap was planned and the location of the main perforator is appropriate, which means that the distance between the perforator and the defect does not exceed the length of the defect diameter, we decided to perform a propeller flap. Otherwise, we considered making freestyle perforator-pedicled or random pattern flap. According to Brunetti et al., the distance between the main perforator and the defect was additionally included as a decisive criterion. If the distance of the audible perforator and the defect did not exceed the size of the defect diameter, they preferred to perform perforator-based propeller flaps. Otherwise, they have used freestyle V-Y advancement perforator-pedicled flaps, which, in their opinion, offered significant advantages, in terms of vascular safety and esthetic outcome, compared to perforator-based propeller flaps [17].
Additional use of hand-held Doppler helped us to locate perforators or recipient vessels for microsurgical anastomosis. Most of the surgical teams use hand-held Doppler and only a few of them the angioscanner (CT). They report the accuracy for locating perforating vessels of approximately 80%. In the literature, we did not find any reports of using conventional angiography for this particular purpose [12, 18, 19].
Except for the cases of emergency and patients with already pre-existing diagnostics such as CTA or MRA, we performed a conventional angiography before the surgery, as described above. In our opinion, it is a useful diagnostic tool because the resolution of conventional arterial angiography is much higher than that of CTA or MRA [20]. Furthermore, conventional angiography allows our radiologists to view the images dynamically from different angles as needed by rotating or moving the leg during the examination. This ability is useful in cases with patients who have metal implants or fixators. In case of CTA or MRA, beam scatter or local signal loss frequently appear in the regions with implants. Furthermore, the possibility of re-canalization by angioplasty during the arterial angiography of some obliterated vessels allows enhanced perfusion, thus improving the outcome of the defect therapy.
The appropriate surgical technique depends on local conditions and general risk factors. Our criteria for choosing propeller flap were the presence of perforators and their proper distance to the defect, as mentioned previously, and the good condition or availability of healthy surrounding tissue. However, Cajozzo et al. included in their algorithm also risk factors such as advanced age, general critical conditions, weight-bearing areas, and wide perilesional scar tissue. When the defects were smaller than 4 cm in width and the patient had no risk factors from the list mentioned by Cajozzo et al., ppf was used, and otherwise, ff was performed [9].
Moreover, Gir et al. conducted a systematic review of 15 publications. According to the review, which included 186 cases of ppf, a complication rate was not influenced significantly by factors such as age, gender, different etiologies (posttraumatic, oncological, postoperative, or osteomyelitis), artery of origin (peroneal artery or posterior tibial artery), and flap size of even more than 200 cm2. Only in the cases where the location of defects was on the distal, third of lower extremity, an increased complication rate was noted [21].
The orientation of raised flaps in another direction than the leg axis does not negatively influence the outcome. Transversally oriented freestyle V-Y advancement flaps seem to be a good alternative in small to medium-sized defects [22].
We did not consider older age or presence of diabetes as contra-indications for the usage of perforator-based propeller flaps. Perhaps, the latter could have been the cause of complication (total flap loss) for this one case, where the patient had severe co-morbidities with the BMI of 55.6, diabetes, a history of heavy smoking, and pronounced trauma of surrounding tissue. Hand-held Doppler showed a good perforator located close to the defect. However, during the revision surgery which took place after the loss of ppf, we discovered pronounced arteriosclerosis, despite his relatively young age of 38 and normal findings of angiography. Hence, it is difficult to specify a particular reason for the failure in this case.
Bekara et al. demonstrated in their meta-analysis of 40 articles, representing 428 ppf cases that only three relevant risk factors are contributing to complications: age older than 60 years, presence of diabetes, and arteriopathy [12]. The following factors have no significant influence on poor outcome: smoking status, high blood pressure, acute or posttraumatic case, bone fracture, location of defect on lower leg (distal third versus other locations of the lower leg), surface area of defect greater than 100 cm2, fascia inclusion, and pedicle rotation greater than 120°. However, Brunetti et al. have shown that the reduced angle of rotation played a role in reducing the risk of vascular complication rate and improving the outcome [23].
Wong et al. demonstrated in their study that a perforator with 1 mm of diameter should be approximately 30 mm long to allow free rotation without buckling and suppressing the perfusion [3].
Gir et al. reported the main complications in the cases with ppf were partial flap necrosis, venous congestion, superficial epidermolysis, total flap loss, and hematoma. Overall, the complication rate of the ppf was by 25%; the most common was partial flap necrosis, which constituted 11.3% of the cases, followed by venous congestion (8.1%). The complication rate of ppf cases from the study of Bekara et al. was 25%, most common among them being partial necrosis (10.2%), complete necrosis (3.5%), epidermolysis (3.5%), and transient venous congestion (3%). Secondary surgery was necessary in 6.45% of the cases, and a complete flap survival rate was up to 84.3% of the cases [12, 21].
Based on the findings from other studies, there is a difference between our complication rate and the rates reported from other centers. One possible reason could be the small number of cases operated in our clinic [24]. The other reason for a difference could be the usage of vacuum-assisted dressings on flaps operated in our division. In the meantime, the other studies have better perfusion rates due to application of vacuum-assisted dressings, which further lead to an improvement in survival rates [25]. However, in our center, for the ff cases without a skin island, the proper postoperative monitoring is limited due to VAC dressing. Thus, the impairment of flap perfusion can be easily undetected, and the necessity of urgent revision surgery can be missed; this can be one of the possible further reasons, why such a high loss rate occurred from ff operated in our division.
Furthermore, the other explanation for the noticeably high complication rate in our patient group who had ff operations could be due to patients’ prior health condition. For example, patients who experienced total free flap loses (14.70%) had either a morbid obesity with the BMI of more than 40 or had severe trauma with an Injury Severity Score (ISS) score of more than 16.
Such systemic risk factors may have contributed to the apparent difference in the complication rates between our patient population and results achieved in other centers, where much lower complication rates of ff were reported [24].
An important aspect of the surgical treatment of defects with flaps is the closure and the morbidity of the donor site. Bekara et al. reported that in most of the cases (69.7%) of patients, who had ppf donor site was self-closing, and in 30.3% of the cases, a skin graft was necessary [12]. Nonetheless, Gir et al. presented contrary results: 37.3% of the cases had direct closure, while 62.7% of the cases required skin graft [21]. It is a drawback of local perforator flaps. While they allow covering the defect in “like with like” fashion, in the majority of the cases, the use of more inferior skin graft adjacent to the flap is necessary, thus reducing the esthetical outcome. In our opinion, in cases with pronounced flap thickness without the possibility of simultaneous flap thinning, such as bulky muscular or myocutaneous flaps, there is a risk of thickness mismatch until the proper adjustment in a secondary surgery can be performed. Free fasciocutaneous or adipocutaneous flaps offer probably best esthetical results, assumed they fit perfectly in the defect cavity.
In our experience of performing local pedicle-based flaps, there were no complications in the area of the donor site. However, as mentioned previously, the esthetical results were not optimal due to the closure with skin grafts. We noticed one complication at the donor site after harvesting ff. It was a seroma, which was successfully treated without revision surgery. Also, except the case with the free foot sole transplantation, all but one donor site was closed with primary direct suture. This method offers better esthetical results at the donor site than the skin graft. Thus, this can be considered as an advantage of performing ff.
However, in some literature, authors argue that the ppf offers numerous advantages over other flap techniques, especially over ff, because it requires shorter surgery and hospitalization duration, result in a lower rate of donor-site morbidity, and allow preservation of main vessels, nerves, and muscles, covering in a “like with like” fashion. Because the microsurgical anastomoses of vessels are required, the procedures of ff demand a higher level of surgical skills and the surgical procedures are more complex and time-consuming. Due to these facts, an overall longer hospitalization time after ff surgery is usually required [12, 26,27,28].
Though it is important to recall that ppf or freestyle perforator-based V-Y advancement flaps are relatively contraindicated in midsize or large defects [17]. Besides, the majority of our ff was harvested in an old-school fashion such as muscle flaps or myocutaneous flaps. As a result, our comparison can be biased against ff because only small number of our cases was operated with free perforator flaps. The further advancement of ff by adopting the perforator concept makes the free perforator flaps more attractive. Despite its requirements concerning the excellent surgical skills, profound anatomical knowledge and surgeons’ confidence, free perforator flap, an improved version of ff, is considered more reliable to be operated on complicated and challenging defects. The free perforator flaps allow the donor sites to be more universal and applicable: the donor sites for conventional ff can be intra-operatively modified onto donor sites for the free perforator flaps, offering better preservation of the main vessels and other structures. Thus, conventional procedures of ff are increasingly replaced by the free perforator flaps [29, 30].
Both concepts, the free flap transfer and the transfer of local pedicle-based flap, possess their advantages and disadvantages. Hence, it is not possible to point out a clear favorite among microsurgical techniques in treating lower leg defects thus far [24].
Conclusions
Many authors recommend performing free flaps on defects on the lower leg. However, an increasing number of surgeons prefer to carry out perforator-based propeller flaps on patients who meet the requirements for this procedure.
Based on our experience, we can suggest the efficacy of local perforator-based flaps operated on defects of the knee, lower leg, and foot. In selectively chosen cases with intact perforators and unmodified tissue surrounding the defect, skilled and experienced surgeons consider a perforator-based propeller flap as a good alternative to a free flap. The perforator-based propeller flap has significantly shorter surgery duration and yields significantly lower complication and revision rate. The use of perforator-based propeller flaps suggests the reduction of the postoperative hospitalization time. However, due to a small number of our cases, the results concerning the duration of the postoperative inpatient care were not significant.
Limitation of this study is that only a few cases of local perforator flaps were performed in our department. Also, most of the free flap surgeries performed in our department were the old-fashioned muscular or myocutaneous flaps, and only a few of them were free perforator flaps. Furthermore, our study lacks data describing the exact size of defects or flaps. It can potentially create a bias against free flaps. Lastly, both subjective and objective standardized assessments of esthetical and functional outcomes are absent.
In summary, due to the complexity of the issue, without conducting further research, it is still not possible to point out a clear favorite microsurgical technique for treating defects in lower leg and foot.
References
Pontén B (1981) The fasciocutaneous flap: its use in soft tissue defects of the lower leg. Br J Plast Surg 34(2):215–220
Taylor GI, Palmer JH (1987) The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg 40(2):113–141
Saint-Cyr M, Wong C, Schaverien M, Mojallal A, Rohrich RJ (2009) The perforasome theory: vascular anatomy and clinical implications. Plast Reconstr Surg 124(5):1529–1544
Hyakusoku H, Yamamoto T, Fumiiri M (1991) The propeller flap method. Br J Plast Surg 44(1):53–54
Oh TS, Hallock G, Hong JP (2012) Freestyle propeller flaps to reconstruct defects of the posterior trunk: a simple approach to a difficult problem. Ann Plast Surg 68(1):79–82
Pignatti M, Ogawa R, Hallock GG, Mateev M, Georgescu AV, Balakrishnan G, Ono S, Cubison TC, D'Arpa S, Koshima I, Hyakusoku H (2011) The "Tokyo" consensus on propeller flaps. Plast Reconstr Surg 127(2):716–722
Blondeel PN, van Landuyt KHI, Monstrey SJM et al (2003) The "gent" consensus on perforator flap terminology: preliminary definitions. Plast Reconstr Surg 112(5):1378–1383; quiz 1383, 1516; discussion 1384-7
Blondeel PN, van Landuyt K, Hamdi M, Monstrey SJ (2003) Perforator flap terminology: update 2002. Clin Plast Surg 30(3):343–346 v
Cajozzo M, Toia F, Innocenti A et al (2017) Retrospective analysis in lower limb reconstruction: propeller perforator flaps versus free flaps. J Reconstr Microsurg 33(S 01):S34–S39
Heller L, Levin LS (2001) Lower extremity microsurgical reconstruction. Plast Reconstr Surg 108(4):1029–1041
Kroll SS, Rosenfield L (1988) Perforator-based flaps for low posterior midline defects. Plast Reconstr Surg 81(4):561–566
Bekara F, Herlin C, Mojallal A, Sinna R, Ayestaray B, Letois F, Chavoin JP, Garrido I, Grolleau JL, Chaput B (2016) A systematic review and meta-analysis of perforator-Pedicled propeller flaps in lower extremity defects: identification of risk factors for complications. Plast Reconstr Surg 137(1):314–331
Yasir M, Wani AH, Zargar HR (2017) Perforator flaps for reconstruction of lower limb defects. World J Plast Surg 6(1):74–81
Tang M, Mao Y, Almutairi K, Morris SF (2009) Three-dimensional analysis of perforators of the posterior leg. Plast Reconstr Surg 123(6):1729–1738
Lazzeri D, Huemer GM, Nicoli F, Larcher L, Dashti T, Grassetti L, Li Q, Zhang Y, Spinelli G, Agostini T (2013) Indications, outcomes, and complications of pedicled propeller perforator flaps for upper body defects: a systematic review. Arch Plast Surg 40(1):44–50
Panse N, Sahasrabudhe P (2014) Free style perforator based propeller flaps: simple solutions for upper extremity reconstruction! Indian J Plast Surg 47(1):77–84
Brunetti B, Tenna S, Aveta A, Segreto F, Persichetti P (2013) Free-style local perforator flaps: versatility of the v-y design to reconstruct soft-tissue defects in the skin cancer population. Plast Reconstr Surg 132(2):451–460
Rand RP, Cramer MM, Strandness DE (1994) Color-flow duplex scanning in the preoperative assessment of TRAM flap perforators: a report of 32 consecutive patients. Plast Reconstr Surg 93(3):453–459
Chang S-M, Zhang F, Xu D-C, Yu GR, Hou CL, Lineaweaver WC (2007) Lateral retromalleolar perforator-based flap: anatomical study and preliminary clinical report for heel coverage. Plast Reconstr Surg 120(3):697–704
Hingorani A, Ascher E, Marks N, Mutyala M, Shiferson A, Flyer M, Jacob T (2007) Comparison of computed tomography angiography to contrast arteriography for patients undergoing evaluation for lower extremity revascularization. Vasc Endovasc Surg 41(2):115–119
Gir P, Cheng A, Oni G, Mojallal A, Saint-Cyr M (2012) Pedicled-perforator (propeller) flaps in lower extremity defects: a systematic review. J Reconstr Microsurg 28(9):595–601
Brunetti B, Poccia I, Tenna S, Campa S, Persichetti P (2015) Transversally oriented pedicled perforator flaps: a reliable alternative for lower leg reconstruction. Microsurgery 35(7):541–545
Brunetti B, Tenna S, Poccia I, Persichetti P (2017) Propeller flaps with reduced rotational angles: clinical experience on 40 consecutive reconstructions performed at different anatomical sites. Ann Plast Surg 78(2):202–207
Rodriguez-Collazo E, Khan A, DiPierro D et al (2018) A systematic review of outcomes and flap selection following lower extremity free tissue transfer versus vascularized perforator pedicle flap transfer in lower limb reconstruction. Int J Orthoplastic Surg 1(2):1–12
Muenchow S, Horch RE, Dragu A (2019) Effects of topical negative pressure therapy on perfusion and microcirculation of human skin. Clin Hemorheol Microcirc. https://doi.org/10.3233/CH-180536
Pinsolle V, Reau AF, Pelissier P et al (2006) Soft-tissue reconstruction of the distal lower leg and foot: are free flaps the only choice? Review of 215 cases. J Plast Reconstr Aesthet Surg 59(9):912–917; discussion 918
Basheer MH, Wilson SM, Lewis H et al (2008) Microvascular free tissue transfer in reconstruction of the lower limb. J Plast Reconstr Aesthet Surg 61(5):525–528
Jakubietz RG, Jakubietz MG, Gruenert JG, Kloss DF (2007) The 180-degree perforator-based propeller flap for soft tissue coverage of the distal, lower extremity: a new method to achieve reliable coverage of the distal lower extremity with a local, fasciocutaneous perforator flap. Ann Plast Surg 59(6):667–671
Kim JT, Kim SW (2015) Perforator flap versus conventional flap. J Korean Med Sci 30(5):514–522
Pribaz JJ, Chan RK (2010) Where do perforator flaps fit in our armamentarium? Clin Plast Surg 37(4):571–579, xi
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Adam Stepniewski, Dominik Saul, Helen Synn, and Gunther Felmerer declare that they have no conflict of interest.
Ethical approval
No ethical approval was needed for this retrospective anonymized evaluation. The use of data was performed in accordance with WMA Declaration of Helsinki.
Informed consent
Informed patient consent was obtained.
Funding
Open Access funding provided by Projekt DEAL.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stepniewski, A., Saul, D., Synn, H. et al. Surgical defect reconstructions in knee, lower leg, and foot with flaps: a retrospective analysis. Eur J Plast Surg 43, 425–434 (2020). https://doi.org/10.1007/s00238-019-01619-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-019-01619-6