Abstract
Preoperative computerised tomographic angiography (CTA) in free flap breast reconstruction outlines the deep inferior epigastric perforator (DIEP). It can identify a single or twin system, measure vessel calibre, and identify iatrogenic/congenital anatomical variations. Evidence of the effect of previous abdominal incisions on this vessel system remain inconclusive. We present the case of a congenital absence of the system identified from routine preoperative CTA. A 61-year-old female presented for immediate unilateral breast reconstruction following mastectomy for ductal carcinoma in situ. She previously had a right-sided Kocher’s incision performed for an open cholecystectomy and a gridiron incision. Coincidentally, preoperative CTA demonstrated congenital absence of the left DIEP system, and marked atrophy of the upper right rectus abdominis (RA). As a result, the superior gluteal artery perforator (SGAP) flap was planned. Intraoperatively, a 1.318-kg SGAP was raised on three perforators and coupled to the internal mammary vessels. In the postoperative period, there were no complications. Preoperative CTA demonstrated multiple hazards in raising a DIEP flap, CTA permitted surgical planning, improving patient safety and surgical efficiency. Preoperative CTA identifies vessel abnormalities resulting from abdominal scarring. The rate of congenital anomalies is unknown. This incidental discovery highlights the role CTA has in reducing operative time, facilitating a successful free tissue transfer, therefore improving patient safety.
Level of Evidence: Level V, diagnostic study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preoperative computerised tomographic angiography (CTA) is routinely practised in our institute, to aid surgical planning in performing the deep inferior epigastric arterial perforating (DIEP) flap for breast reconstruction. A dominant perforator is selected based upon vessel diameter, position, separation and end terminal branches. There is a well-published variability in the anatomy of the DIEP describing multiple classifications in addition to the possibility of previous surgical incisions altering these dependable yet varied structures [1].
Mateucci et al. discussed the iatrogenic absence of the DIEP in a patient who received a Wertheim’s hysterectomy [2]. However, DIEP system absence has only previously been documented in a virgin abdomen by Rozen et al. There are no published reports at all on the relationship between Kocher’s incisions and superior epigastric artery/DIEP system alterations [3].
We introduce the second case of a congenitally absent DIEP system and the presence of a Kocher incision, complicating preoperative planning in breast reconstruction.
Case report
We present the case of a 61-year-old woman who underwent immediate reconstruction of the right breast. She had a body mass index of 37 and has a breast size G cup. On examination, the patient had abdominal scars from a Kocher incision and a gridiron incision. She initially presented to the breast surgery department after a screen detected breast lesion. A diagnosis of intraductal papilloma and ductal carcinoma in situ (DCIS) of the right breast was made. She received a wide local excision, which had involvement of a margin with low-grade DCIS. Therefore, a right total mastectomy and immediate breast reconstruction was performed.
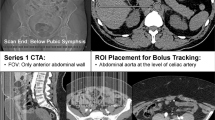
The most common method of autologous reconstruction in our department, considering patient preference, is the immediate DIEP. Preoperative CTA observed an intact right DIEP system but a congenital absence of the left DIEP system (Fig. 1). The upper portion of the right rectus abdominis (RA) and the surrounding soft tissue were atrophic. This relates to surgical damage of the superior epigastric artery and nerve supply. At the level of the umbilicus, the thickness of the left RA was 7.06 mm but absent on the right as muscle density was lower suggesting it was entirely replaced with fat. At the level of the umbilicus, the right RA was 10.22 mm thick while the left RA was 15.00 mm. Below the umbilicus, the RA is smaller by a third on the right compared to the left side. There were two sets of perforators bilaterally, the left medial row perforator was 2.31 mm which quickly merged into the left deep superior epigastric artery (DSEA) but did not communicate with the left deep inferior epigastric artery (DIEA), and the right medial row perforator was 2.04 mm. The DSEA was bilaterally atrophic and a superifical inferior epigastric artery and vein were present but were not dilated on the right side of the abdomen.
The absence of the left DIEA, with no signs of previous trauma or surgical alterations in this region of the abdomen, leads to the conclusion that it must be congenital in origin. There was obvious concern regarding the use of a DIEP flap, not relating to elevation of a right-sided flap, but in relation to the remaining vascular anatomy for abdominal wall closure. The same angiogram was used to provide an alternative reconstruction; a superior gluteal artery perforator (SGAP) flap was used as the donor site was sufficient for autologous transfer. Intraoperatively, the right SGAP flap was raised on three perforators and anastomosed to the right internal mammary vessels. The final weight of the flap was 1.318 kg and the total ischaemia time was 1 h and 20 min without any intra- or postoperative complications.
Discussion
We report the second case of a congenitally absent DIEP system which has only previously been reported once by Rozen et al. who reported the complete absence of a unilateral DIEA [3]. In Rozen et al., the ipsilateral DSEA was dilated and communicated with the umbilical perforators [3]. In this case, the origin of the left DIEA was observed originating from the external iliac artrey, but atrophies in the lower RA without communicating with the DSEA (Fig. 2).
A DIEP flap would not have been successful as dissection of the left umbilical perforator would result in the discovery that the vessel calibre does not increase and does not communicate with the left DIEA, resulting in an abortive operation. A DIEP can be performed in patients with a Kocher scar using a technical modification in closure of the abdomen [4]. However, in this insistance, raising a right DIEP or TRAM would have resulted in further ischaemia of the RA leading to potential flap loss and umbilical necrosis due to the absent left DIEA combined with the bilaterally atrophic DSEAs. Closure of the abdomen would have been precarious as the remaining blood supply to the anterior abdominal wall would be from the external oblique perforators, of which some may require division to facilitate the abdominoplasty closure and the circumflex iliac system, of which the superficial system may occasionally be ligated in elevation of the DIEP flap.
In addition, patients with a Kocher scar are a high-risk group for a DIEP as adjuvant treatment for breast malignancy may be delayed by poor wound healing which there is an increased predisposition to in patients with abdominal scarring [5]. A SIEA was not harvested as this was located 6 cm inferior and lateral to the anterior superior iliac spine, due to the patient’s body habitus, making a standard abdominoplasty harvest difficult.
It is particularly important that patients with abdominal scars should have CTA relating to the well-documented risk they impose upon the DIEP. However, the use of CTA allows DIEP procedures to be offered to suitable candidates with abdominal scarring, rather than withholding the procedure based on the clinical picture, as is the case in some other units. Although small, the risk of discovering either a congenital or scar-induced abnormality can be reduced with preoperative CTA.
References
Ireton JE, Lakhiani C, Saint-Cyr M (2014) Vascular anatomy of the deep inferior epigastric artery perforator flap: a systematic review. J Plast Reconstr Aesthet Surg 132(5):810e–821e
Matteucci P, Stanley PR, Bates J, Riaz M (2009) Complete absence of lower rectus abdominus muscle and deep inferior epigastric artery complicating free DIEP flap breast reconstruction. J Plast Reconstr Aesthet Surg 62:e112–e113
Rozen WM, Houseman ND, Ashton MW (2009) The absent inferior epigastric artery: a unique anomaly and implications for deep inferior epigastric artery perforator flaps. J Reconstr Microsurg 25:289–293
Schoeller T, Huemer GM, Kolehmainen M, Otto-Schoeller A, Wechselberger G (2004) Management of subcostal scars during DIEP-flap raising. Br J Plast Surg 57(6):511–514
Shermak MA, Mallalieu J, Chang D (2010) Do preexisting scars threaten wound healing in abdominoplasty? Eplasty 10:e14
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
Thomas G. W. Harris, Helen S. Wohlgemut, Gerald Lip and Alain Pierre Robert Curnier declare that they have no conflict of interest.
Ethical approval
For this kind of study formal consent from a local ethics committee is not required.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Harris, T.G.W., Wohlgemut, H.S., Lip, G. et al. Congenital absence of the deep inferior epigastric system: a case report. Eur J Plast Surg 42, 197–200 (2019). https://doi.org/10.1007/s00238-018-1463-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-018-1463-7