Abstract
Purpose
To investigate pain hypervigilance in individuals suffering from chronic neck and shoulder pain (CNSP) and its underlying brain mechanism.
Methods
The evaluation of pain vigilance was conducted through the utilization of pain vigilance and awareness questionnaires. Voxel-wise regional homogeneity (ReHo) from 60 CNSP patients and 60 healthy controls (HCs) using resting-state fMRI data. Voxel-wise two-sample T-test was conducted to reveal the ReHo variations between CNSP and HC. Correlation analyses were utilized to reveal the connection between brain abnormalities and medical measurements. Furthermore, a mediation analysis was conducted to elucidate the pathway-linking changes in brain function with medical measurements.
Results
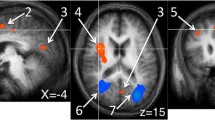
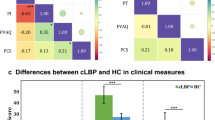
Our present study revealed three main findings. Firstly, patients with CSNP demonstrated a heightened vigilance of pain in comparison to healthy adults, a common occurrence among individuals with chronic pain conditions. Secondly, we observed brain abnormalities in various brain regions in CSNP patients, and these alterations were associated with the extent of pain vigilance. Lastly, the pain hypervigilance impact on the severity of pain was found to be controlled by regional neural activity in the anterior cingulate cortex (ACC) in subjects with CSNP.
Conclusion
Our findings suggested that long-term repetitive nociceptive input caused by chronic pain further aggravates the pain intensity by impairing the vigilance-related pain processing within the anterior cingulate cortex in CNSP patients.



Similar content being viewed by others
Data availability
The data and the codes used in current study are available upon reasonable request to the corresponding author.
References
Cao S et al (2021) Clinical efficacy and safety of “three-dimensional balanced manipulation” in the treatment of cervical spondylotic radiculopathy by finite element analysis. Biomed Res Int 2021:5563296
Luyao H et al (2022) Management of cervical spondylotic radiculopathy: a systematic review. Global Spine J 12(8):1912–1924
Truumees E, Herkowitz HN (2000) Cervical spondylotic myelopathy and radiculopathy. Instr Course Lect 49:339–360
Alshelh Z et al (2022) Neuroimmune signatures in chronic low back pain subtypes. Brain 145(3):1098–1110
Baliki MN et al (2012) Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 15(8):1117–1119
Li T et al (2022) Functional connectivity modulations during offset analgesia in chronic pain patients: an fMRI study. Brain Imaging Behav 16(4):1794–1802
Malfliet A et al (2017) Brain changes associated with cognitive and emotional factors in chronic pain: a systematic review. Eur J Pain 21(5):769–786
Tu Y et al (2021) Magnetic resonance imaging for chronic pain: diagnosis, manipulation, and biomarkers. Sci China Life Sci 64(6):879–896
Moore DJ, Keogh E, Eccleston C (2009) Identifying experimental methods to determine the effect of pain on attention: a review of pain, caffeine, alcohol and nicotine studies. Hum Psychopharmacol 24(8):601–618
Torta DM et al (2017) Attention to pain! A neurocognitive perspective on attentional modulation of pain in neuroimaging studies. Cortex 89:120–134
Van Damme S et al (2010) Keeping pain in mind: a motivational account of attention to pain. Neurosci Biobehav Rev 34(2):204–213
Lister KC et al (2020) Chronic pain produces hypervigilance to predator odor in mice. Curr Biol 30(15):R866-r867
Louw A et al (2011) The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain. Arch Phys Med Rehabil 92(12):2041–2056
Meints SM, Edwards RR (2018) Evaluating psychosocial contributions to chronic pain outcomes. Prog Neuropsychopharmacol Biol Psychiatry 87(Pt B):168–182
Heathcote LC, Simons LE (2020) Stuck on pain? Assessing children’s vigilance and awareness of pain sensations. Eur J Pain 24(7):1339–1347
Monticone M et al (2016) Development of the Italian version of the Pain Vigilance and Awareness Questionnaire in subjects with chronic low back pain: cross-cultural adaptation, confirmatory factor analysis, reliability, and validity. Int J Behav Med 23(2):214–223
Steinberg SN et al (2022) Within-individual BOLD signal variability in the N-back task and its associations with vigilance and working memory. Neuropsychologia 173:108280
Wong WS, McCracken LM, Fielding R (2011) Factorial validity and reliability of the Chinese version of the Pain Vigilance and Awareness Questionnaire (ChPVAQ) in a sample of Chinese patients with chronic pain. Pain Med 12(7):1018–1025
Goossens N et al (2019) Differences in brain processing of proprioception related to postural control in patients with recurrent non-specific low back pain and healthy controls. Neuroimage Clin 23:101881
Medrano-Escalada Y et al (2022) Structural, functional and neurochemical cortical brain changes associated with chronic low back pain. Tomography 8(5):2153–2163
Luo Y et al (2016) Altered neural correlates of emotion associated pain processing in persistent somatoform pain disorder: an fMRI Study. Pain Pract 16(8):969–979
Otti A et al (2013) Functional network connectivity of pain-related resting state networks in somatoform pain disorder: an exploratory fMRI study. J Psychiatry Neurosci 38(1):57–65
Yoshino A et al (2013) Distinctive neural responses to pain stimuli during induced sadness in patients with somatoform pain disorder: an fMRI study. Neuroimage Clin 2:782–789
Dai H et al (2020) A combined DTI and resting state functional MRI study in patients with postherpetic neuralgia. Jpn J Radiol 38(5):440–450
Xiang A et al (2021) Frequency-specific blood oxygen level dependent oscillations associated with pain relief from ankle acupuncture in patients with chronic low back pain. Front Neurosci 15:786490
Yue X, Du Y (2020) Altered intrinsic brain activity and regional cerebral blood flow in patients with chronic neck and shoulder pain. Pol J Radiol 85:e155–e162
Chen J et al (2018) Regional homogeneity and multivariate pattern analysis of cervical spondylosis neck pain and the modulation effect of treatment. Front Neurosci 12:900
Yu CX et al (2017) Abnormality of spontaneous brain activities in patients with chronic neck and shoulder pain: a resting-state fMRI study. J Int Med Res 45(1):182–192
Iannetti GD, Mouraux A (2010) From the neuromatrix to the pain matrix (and back). Exp Brain Res 205(1):1–12
Legrain V et al (2011) The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol 93(1):111–124
Mouraux A et al (2011) A multisensory investigation of the functional significance of the “pain matrix.” Neuroimage 54(3):2237–2249
Salomons TV et al (2016) The “pain matrix” in pain-free individuals. JAMA Neurol 73(6):755–756
De Pauw R et al (2020) Hub disruption in patients with chronic neck pain: a graph analytical approach. Pain 161(4):729–741
Yu CX et al (2017) Altered functional connectivity of the periaqueductal gray in chronic neck and shoulder pain. NeuroReport 28(12):720–725
Li H et al (2020) Deficits in ascending and descending pain modulation pathways in patients with postherpetic neuralgia. Neuroimage 221:117186
Gong W et al (2022) The subsequent interruptive effects of pain on attention. Eur J Pain 26(4):786–795
Lenz FA, Treede RD (2002) Attention, novelty, and pain. Pain 99(1–2):1–3
Ito H et al (2023) Chronic pain recruits hypothalamic dynorphin/kappa opioid receptor signalling to promote wakefulness and vigilance. Brain 146(3):1186–1199
Aldrich S, Eccleston C, Crombez G (2000) Worrying about chronic pain: vigilance to threat and misdirected problem solving. Behav Res Ther 38(5):457–470
Kunz M et al (2017) Psychometric properties of the German version of the Pain Vigilance and Awareness Questionnaire (PVAQ) in pain-free samples and samples with acute and chronic pain. Int J Behav Med 24(2):260–271
Nagasaka K et al (2022) Cortical signature related to psychometric properties of pain vigilance in healthy individuals: a voxel-based morphometric study. Neurosci Lett 772:136445
Roelofs J et al (2003) The Pain Vigilance and Awareness Questionnaire (PVAQ): further psychometric evaluation in fibromyalgia and other chronic pain syndromes. Pain 101(3):299–306
Van Bogaert W, Coppieters I, Kregel J, Nijs J, De Pauw R, Meeus M, Cagnie B, Danneels L, Malfliet A (2021) Influence of baseline kinesiophobia levels on treatment outcome in people with chronic spinal pain. Phys Ther 101(6):pzab076. https://doi.org/10.1093/ptj/pzab076
Song Y et al (2021) Feedforward and feedback pathways of nociceptive and tactile processing in human somatosensory system: a study of dynamic causal modeling of fMRI data. Neuroimage 234:117957
Zhao R et al (2022) Brain-activation-based individual identification reveals individually unique activation patterns elicited by pain and touch. Neuroimage 260:119436
Hung PS et al (2022) Differential expression of a brain aging biomarker across discrete chronic pain disorders. Pain 163(8):1468–1478
Kumbhare DA, Elzibak AH, Noseworthy MD (2017) Evaluation of chronic pain using magnetic resonance (MR) neuroimaging approaches: what the clinician needs to know. Clin J Pain 33(4):281–290
Ossipov MH, Morimura K, Porreca F (2014) Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 8(2):143–151
Zhao Y et al (2022) Neuroimaging studies of chronic prostatitis/chronic pelvic pain syndrome. Pain Res Manag 2022:9448620
Labrakakis C (2023) The role of the insular cortex in pain. Int J Mol Sci 24(6):5736. https://doi.org/10.3390/ijms24065736
Apkarian AV et al (2005) Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9(4):463–484
Lee JA, Chen Q, Zhuo M (2022) Synaptic plasticity in the pain-related cingulate and insular cortex. Biomedicines 10(11):2745. https://doi.org/10.3390/biomedicines10112745
Lu C et al (2016) Insular cortex is critical for the perception, modulation, and chronification of pain. Neurosci Bull 32(2):191–201
Mutschler I et al (2012) Pain and emotion in the insular cortex: evidence for functional reorganization in major depression. Neurosci Lett 520(2):204–209
Nieuwenhuys R (2012) The insular cortex: a review. Prog Brain Res 195:123–163
Wang N, Zhang YH, Wang JY, Luo F (2021) Current understanding of the involvement of the insular cortex in neuropathic pain: a narrative review. Int J Mol Sci 22(5):2648. https://doi.org/10.3390/ijms22052648
Zhuo M (2016) Contribution of synaptic plasticity in the insular cortex to chronic pain. Neuroscience 338:220–229
Hwang UJ, Kwon OY (2022) Influencing factors of pressure pain hypersensitivity of the upper trapezius in food service workers with nonspecific neck/shoulder myofascial pain: a cross-sectional study. Medicine 101(31):e29696
Afif A et al (2008) Middle short gyrus of the insula implicated in pain processing. Pain 138(3):546–555
Bai L, Zhang L, Chen Y, Li Y, Ma D, Li W, Meng Y, Zhao Y, Wang Y, Zeng Q, Zhuang Q (2022) Middle cingulate cortex function contributes to response to non-steroidal anti-inflammatory drug in cervical spondylosis patients: a preliminary resting-state fMRI study. Neuroradiology 64(7):1401–1410. https://doi.org/10.1007/s00234-022-02964-3. (Erratum in: Neuroradiology 2022 May 27)
Bliss TV et al (2016) Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 17(8):485–496
Xiao X, Ding M, Zhang YQ (2021) Role of the anterior cingulate cortex in translational pain research. Neurosci Bull 37(3):405–422
Wang YQ et al (2021) Neuropathic pain generates silent synapses in thalamic projection to anterior cingulate cortex. Pain 162(5):1322–1333
Li XH et al (2021) Oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional anxiety by inhibiting presynaptic long-term potentiation. Cell Rep 36(3):109411
Smith ML, Asada N, Malenka RC (2021) Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science 371(6525):153–159
Vaccarino AL, Melzack R (1989) Analgesia produced by injection of lidocaine into the anterior cingulum bundle of the rat. Pain 39(2):213–219
Tsuda M et al (2017) Neuronal and microglial mechanisms for neuropathic pain in the spinal dorsal horn and anterior cingulate cortex. J Neurochem 141(4):486–498
Kuchinad A et al (2007) Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci 27(15):4004–4007
Funding
This study has received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Institutional Review Board of Tianjin Hospital approval was obtained.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Wang, H. & Guo, L. Investigating the brain functional abnormalities underlying pain hypervigilance in chronic neck and shoulder pain: a resting-state fMRI study. Neuroradiology (2024). https://doi.org/10.1007/s00234-024-03286-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00234-024-03286-2