Abstract
The central nervous system (CNS) undergoes constant immune surveillance enabled via regionally specialized mechanisms. These include selectively permissive barriers and modifications to interlinked innate and adaptive immune systems that detect and remove an inciting trigger. The end-points of brain injury and edema from these triggers are varied but often follow recognizable patterns due to shared underlying immune drivers. Imaging provides insights to understanding these patterns that often arise from unique interplays of infection, inflammation and genetics. We review the current updates in our understanding of these intersections and through examples of cases from our practice, highlight that infection and inflammation follow diverse yet convergent mechanisms that can challenge the CNS in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The inciting triggers for brain injury can be varied. However, these often converge on common mechanistic end-points due to similarities in underlying immune drivers. Our understanding of the initiating event (such as an underlying antibody or infection), subsequent immune machinery in play and eventual pathology of the generated insult has considerably expanded in recent times. The COVID-19 pandemic has further provided opportunities to study the interactions between infectious triggers and immune-CNS interfaces. Through this review article, we aim to provide updates in our understanding of central nervous system (CNS) immune mechanisms and explore imaging similarities and differences across multiple acquired and genetic disorders. We highlight that in children, CNS infection and inflammation often follow diverse yet unifying pathways.
T cell gateways—brain barriers and T cell entry pathways
The understanding of CNS and immune system interactions have evolved considerably. We provide a brief overview and select updates in blood–brain barrier (BBB) pathways. Prior perceptions of an “immune-privileged” brain stemmed from a traditional understanding of the blood–brain “barrier” as an anatomical non-permissive barrier. The underpinnings behind this concept have been challenged with time and CNS immune-privilege has been redefined [1]. The first barrier protecting the brain from the external environment is the skull vault. Persistence of embryological pathways can compromise and bypass this barrier, forming routes of spread of infection or can lead to direct CNS inflammation (Fig. 1). Within the brain parenchyma, the BBB forms a stable neurovascular unit tuned to respond to signals from the CNS innate machinery and screen for systemic immune cells and potential pathogens. Under physiological conditions, tight junction proteins in BBB block T cell entry which are thus virtually absent from the brain parenchyma [2, 3]. When summoned via innate immune responses, the endothelial cells of BBB undergo a series of non-disruptive (and eventually disruptive) changes that enable T cell entry, and can also double up as antigen presenting cells (APCs) to control local adaptive immune responses [2, 4, 5]. However, these BBB properties are not absolute, vary with age and are even regionally different across brain interfaces with blood-CSF, blood-meninges barriers being more efficient in trafficking T cells [2, 3]. Defects in the BBB and lymphocyte trafficking in “early” MS and seemingly BBB-independent mechanisms of self-sustained CNS inflammation in the “later” stages have emerged as the leading hypothesis in MS [6].
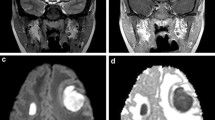
Epidermoid cyst and chemical meningitis in a 3-year-old child. Sagittal T2 (A), post-contrast T1 (B), axial DWI (C), and ADC maps (D), sagittal and axial CT reformats (E, F) images. MR images (A–D) show a midline mass centered at the anterior skull base and projecting towards to basifrontal lobe with heterogenous T2 signal (A), nodular and rim enhancement (dashed and solid arrows (B), diffusion restriction within the lesion (arrow C), and surrounding vasogenic edema (arrow D). Possibilities of an abscess and congenital ectodermal inclusion cyst were raised. CT done to assess bony anatomy shows a widened remodelled foramen cecum at the anterior skull base (arrows E, F). Intraoperatively, this was confirmed to be a ruptured epidermoid cyst precipitating a chemical meningitis
T cell gateways—meningeal lymphatics and T cell exit pathways
Recent work on CNS lymphatic networks has provided further insights into T cell trafficking. Interlinked discoveries over the last decade have confirmed a synergistic clearance system between the paravascular space (glymphatic system) and the meningeal lymphatic vessels [7,8,9]. Brain lymphatic vessels are harboured within the meninges instead of the parenchyma [10]. The parenchyma instead utilizes a brain-wide network of CSF-ISF exchange housed in the paravascular space (between the glia limitans and vascular endothelial membrane, termed the glymphatic network) to clear waste products (Fig. 2). While major mechanistic drivers of this system remain under debate, Aquaporin 4 channels serve a sentinel role in regulating these conduits [11, 12]. Further downstream, the meningeal lymphatic vessels (MLVs) form the major route of transdural CSF efflux and macromolecular clearance. They also serve as T cell “exit pathways,” draining them to deep cervical lymph nodes where they can further recruit systemic immune cells against CNS antigens (Fig. 2) [13, 14]. Evidence for their neuroimmunological role is mounting [7, 9, 15]. More recently, MLVs have also been implicated in CNS virus drainage and clearance in animal models, and accelerated recovery from infections in mice models has been noted with treatments promoting MLV expansion [16].
Glymphatic network, meningeal lymphatic vessels, and dorsal transdural CSF efflux. CSF efflux can occur via transvenous and transdural routes. We depict the major route of dorsal transdural CSF efflux — the meningeal lymphatic vessels and the functionally linked glymphatic network. The glymphatic network consists of paravascular spaces (PVS) that surround the pial and parenchymal arteries and veins, and forms a continuum with the subarachnoid space (SAS). This allows CSF to communicate with the parenchymal interstitial fluid (ISF, solid small green arrows) via channels in the glia limitans, essential for solute and fluid exchange. The ISF drains the parenchymal waste products (amyloid β, lactate, other macromolecules) via perivenous paravascular spaces into the subarachnoid CSF (dashed small green arrows), thus ensuring CSF-ISF homeostasis. The parasagittal dural meningeal lymphatic vessels (MLVs) form the major dural efflux pathways and enable further downstream clearance of macromolecules. They also play a role in immunomodulation by trafficking immune cells (curved large solid green arrows) directly to the deep cervical lymph nodes. Ventral pathways (along the major cranial nerves, skull base foramina, and olfactory fossa) form the other routes of transdural CSF efflux
MLV and glymphatic network lack direct physical connections but evidence suggests that these compartments are functionally linked [17]. Just like the BBB, the glymphatics undergo senescence with implications on cognition as well as neurodegenerative disorders, stroke and normal pressure hydrocephalus [9]. However, much remains unknown how this may contribute to CNS inflammation, particularly in the pediatric context. This has nonetheless paved way for newer pathways in understanding CNS-immune crosstalk in neuroinflammation.
Innate and adaptive immunity — bridges between infection and neuroinflammation, and hygiene/old friends hypothesis
The immune system has two interlinked subsystems with specific molecular and cellular effectors that act synergistically to produce the end-product of inflammation. This includes an evolutionarily conserved innate immune system based on non-specific pattern recognition approaches that identify a broad category of infections, and an adaptive immune system which is armed to produce pathogen specific cascades. We discuss these in the context of CNS inflammation.
As a first responder, pathogen sensing in innate immunity occurs via pattern recognition receptors (PRRs), which recognize broad sequences in bacteria and viruses termed pathogen-associated molecular patterns (PAMPs) that are common across these pathogens. PRRs can also recognize endogenous signals in “sterile brain inflammation” via damage associated molecular patterns (DAMPs) [18]. PRRs include a large family of receptors, the best known being the Toll-like receptors (TLRs) which are highly expressed on microglia, the sentinel resident CNS innate immune cell. Binding of PAMPs to their respective TLR leads to microglial activation. This leads to downstream production of chemokines and cytokines, and initiation of signaling pathways including interferons and complement cascade. Certain cytokines, particularly IL-12, subsequently enable a transition to adaptive immune response by increasing BBB permeability and recruitment of circulating T and B lymphocytes [19, 20]. We discuss some of the specialized pathways of adaptive immunity under the section “Defects of perforin-dependent pathway.”
The degrees of activation of the two subsystems and roles further downstream are highly variable and governed by numerous environmental and host factors. Also, genetic variants and polymorphisms may confer susceptibility to specific agents. For example, genetic variants causing TLR3 deficiency or defects in TLR3 signaling pathway play a well-established role in susceptibility to HSV encephalitis (HSVE) [21]. Finally, epidemiological studies lend support to a poorly understood mechanism of immune remodelling. Termed the hygiene/old friends hypothesis, this suggests that human immune system evolved with ancient gut microbes and soil-based helminths over millions of years modulating immune system responses. This has potential repercussions on immune system-gut microbiome interactions and may even shed light on dysregulated hyperinflammatory states in SARS-CoV-2 [22, 23].
Patterns of edema and pediatric CNS injury—cytokines, vascular compromise, and excitotoxicity
Brain edema is a manifestation of parenchymal injury, especially in the context of infection and inflammation. The patterns of parenchymal edema are often recurrent and lead to recognizable MRI phenotypes based on either specific signal changes on conventional sequences (cytotoxic, vasogenic, intramyelinic) or a distinct topography (excitotoxic, osmotic, intramyelinic, interstitial – Fig. 3). Topographical patterns are also well described in the context of pediatric viral infections and recognizable patterns are likely due to common immunological determinants [24]. Here, we discuss three entities with distinct patterns of brain edema and highlight the divergent underlying immune mechanisms—Parechovirus encephalitis, cerebral malaria, and claustral injury.
Types of cerebral edema. Cerebral edema can arise from distinct etiological mechanisms including vascular (PRES, infarction), osmolar (myelinolysis), interstitial (increased ventricular pressures), myelin vacuolization (megalencephalic leukoencephalopathy with subcortical cysts or MLC), and excitatory (intramyelinic edema) often forming recognizable patterns of topographical distribution and signal change
Parechovirus encephalitis
One of the unique patterns of white matter injury seen during the neonatal period, first described with human Parechovirus 3 (HPeV3) [25] and subsequently with enterovirus (EV), rotavirus, Chikungunya, and in hyperinflammatory states in SARS-CoV-2, is characterized by a striking radiating pattern of restricted diffusion involving the periventricular and deep white matter often with a frontal predominance (Fig. 4) [26,27,28]. Evolution is typically into gliosis or a cystic PVL-like pattern [26]. Involvement of long tracts of corpus callosum, optic radiations, internal capsules, and thalami may be seen as well. The mechanisms behind this and the sentinel anatomical substrate of injury are poorly understood but may be due to common immunological signatures across these pathogens and unique vulnerabilities of the neonatal brain [29].
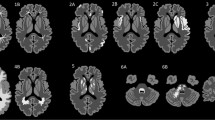
Patterns of cerebral edema in Parechovirus encephalitis and cerebral malaria. Axial DWI T2 (A–D), ADC maps (A′–D′). Case 1: a 10-day-old term baby admitted with fever, irritability and seizures. Diffusion (A, B) and ADC maps (A′, B′) in this case of Parechovirus encephalitis show radiating stripes of diffusion restriction in the periventricular and deep white matter with frontal predominance (arrows) and few smaller foci in the occipito-temporal regions. Diffusion restriction is also noted in the genu and splenium of corpus callosum (dashed arrows). Case 2: a 10-year-old boy with high-grade fever, comatose, and recent travel history. Diffusion (C, D) and ADC maps (C′, D′) in this case of cerebral malaria show subcortical predominant restricted diffusion in the frontoparietal regions bilaterally (arrows C, C′). Diffusion restriction in the splenium is noted as well, likely from excitotoxic intramyelinic edema (arrows, D, D′)
The brain pathology in HPeV3 and above-mentioned viruses has similarities. Direct CNS invasion by T cells and adaptive inflammation is characteristically lacking, also reflected in the absence of pleocytosis, bland CSF picture and low systemic inflammatory markers in HPeV3 encephalitis [30, 31]. Innate immunity via TLRs and activation of microglia appears to be the main driver of inflammation [29, 31]. The imaging pattern also resembles patterns from deep medullary vein engorgement and/or thrombosis, a proposed alternate or synchronous mechanism [32]. However, this is not conclusive from autopsy evidence and susceptibility weighted imaging (SWI) may be normal in many [27, 31].
Cerebral malaria
Cerebral malaria also provides an interesting avenue to study brain edema patterns. Imaging findings are on a spectrum and there are differences between children and adults [33]. The basal ganglia, thalami, white matter and cortex are commonly involved regions [34]. On diffusion weighted imaging (DWI), transient bilateral subcortical white matter restricted diffusion sparing the cortex is a common and characteristic finding in children (Fig. 4) [35]. This can be seen with or without callosal involvement and is associated with better outcomes and good neurological recovery [35]. This pattern is uncommon in adults who instead display a vulnerability of the basal ganglia and thalami [33, 35, 36].
The proposed cascade of events is initiated via microvascular pathology and RBC sequestration which are the pathological hallmarks [37]. This can progress to varying degrees of microvascular occlusion creating regions of hypoperfusion and hypoglycemia. A metabolic failure ensues, aggravated by co-existing factors increasing metabolic demands such as seizures and hyperpyrexia [35]. This leads to disturbances in glutamate cycling and extracellular glutamate excess, excitotoxicity, and intramyelinic edema seen pathologically as myelin vacuolation and is reversible with timely treatment in keeping with its transient nature and better outcomes [35, 37]. In advanced cases, there is progression to microvascular thrombi, perivascular hemorrhages and BBB disruption, eventually culminating in irreversible changes.
Claustral injury and possible immune mechanisms
Another topographically distinct pattern seen on MRI is the selective symmetric signal abnormalities within the claustrum [38]. Claustrum is a thin strip of gray matter flanked by white matter bundles on either side and serves as a major connectomic hub with extensive reciprocal connections with the ipsilateral cortical structures and basal ganglia (Supplementary Fig. 1).
Claustral lesions were reported as a form of immunotherapy responsive injury in viral encephalitis [39, 40]. This description has since expanded to other entities including febrile infection-related epilepsy syndrome (FIRES), autoimmune epilepsy, acute encephalitis with refractory, repetitive partial seizures (AERRPS) in children and as a post-febrile de novo status epilepticus syndrome with reversible lesions in adults [38, 41,42,43]. Novel entities including immune effector cell-associated neurotoxicity syndrome (ICANS) and parainfectious SARS-CoV-2 phenomenon can also have this pattern, possibly linked to a preceding cytokine storm and is termed cytokine storm-associated encephalopathy (CySE) [38, 44]. Immunotherapy responsiveness further supports potential dysregulated cytokine signaling as a common link, although reasons for claustrum predilection in hypercytokinemia are not well understood.
Autoimmune (anti-NMDAR and MOG encephalitis) and viral encephalitis
Infection and autoimmune encephalitis (AE) remain the most common considerations in pediatric encephalitis. In AE, neural autoantibodies can be directed against extracellular (cell-surface or synaptic) or intracellular antigens (most paraneoplastic or onconeural antibodies). Due to logistical challenges in antibody detection, updated diagnostic criteria still focus on conventional tests and the absence of antibody does not exclude AE [45]. Pediatric AE additionally holds unique diagnostic challenges in contrast to adults due to multifocal and atypical presentations rather than well-defined clinical syndromes and overlaps with other conditions unique to pediatric cohorts, necessitating modifications to criteria for pediatric AE [46].
Medial temporal lobe involvement on MRI often leads to search patterns for autoimmune limbic encephalitis and HSVE in children. Anti-NMDAR encephalitis is now the leading cause of AE in children, and more common than any single viral encephalitis [47]. MRI is most commonly normal (~ 65%), followed by a limbic encephalitis pattern (Fig. 5) [46]. Other patterns include striatal encephalitis with or without limbic involvement (Fig. 5), brainstem encephalitis, cerebellitis, or isolated cortical involvement.[48] Despite overlaps, tendency to spare basal ganglia, hemorrhages, and enhancement can help recognize HSVE (Fig. 6) [49]. Also, the presence of diffusion restriction is atypical for anti-NMDAR encephalitis and may warrant search for alternate etiologies or explanations [46].
Anti-NMDAR encephalitis: Case 1: axial FLAIR images (A–D) and Case 2 axial FLAIR (E), axial and coronal T2 (F, G), axial chest CT (H). Case 1: a 12-year-old girl presenting with increased forgetfulness, drop in grades, and bouts of violent behaviour for 3 months. Axial FLAIR images show limbic and striatal involvement. There is hyperintensity involving the hippocampus, amygdala, and parahippocampal gyri bilaterally (arrows A, dashed arrows B). Involvement of the basal ganglia (arrows C, D) and perisylvian regions (arrows B) is noted as well. No diffusion restriction or enhancement was noted. CSF was positive for anti- NMDAR antibodies. Case 2: a 14-year-old girl with mediastinal teratoma and anti-NMDAR encephalitis presenting acutely with 2-day history of behavioural changes. Imaging shows asymmetrical (left > right) but bilateral involvement of the hippocampi (arrows E–G). Chest CT shows a heterogeneously enhancing mass lesion in the anterior mediastinum (arrow, H), biopsy was suggestive of a teratoma
Herpes simplex virus encephalitis (HSVE) and post-HSVE anti-NMDAR encephalitis. Case 1: axial T2 (A, C), axial post-contrast T1 (B), and axial non-contrast CT (D); Case 2: axial FLAIR (E, F, H) and axial DWI (G). Case 1: a 10-year-old with characteristic findings of HSVE including severe swelling and T2 hyperintensity of the right temporal lobe (arrows A) extending to the perisylvian region (arrow C) with mild enhancement (arrow D). Hyperdensity and parenchymal hemorrhage are noted on the CT (arrow D). Case 2: an 8-year-old presenting with fever, seizures, and altered consciousness. There are multifocal areas of parenchymal swelling, FLAIR hyperintensity, and diffusion restriction in right temporal (arrows E, G) and left temporo-occipital lobes (dashed arrows E, G). The right temporal horn is effaced. CSF PCR was positive for HSV. Follow-up imaging 6 weeks later shows gliosis and cystic encephalomalacia (arrows F). Imaging 2 months later (H), now presenting with new movement disturbance, shows extensive confluent FLAIR hyperintensity of the white matter and a leukodystrophy-like picture (asterisks H). CSF was positive for anti-NMDAR antibodies
Limbic encephalitis can be imaging negative, unilateral, or bilateral. However, upgraded criteria for “definite” autoimmune limbic encephalitis require bilateral involvement in the absence of neural autoantibodies to avoid a potential misdiagnosis [45]. MRI also serves to exclude other potential mimics including demyelination, vasculitis, and infectious and parainfectious etiologies in which imaging is more commonly abnormal.
HSVE is also a potent trigger for autoimmunity including anti-NMDAR encephalitis and acquired demyelinating syndromes (ADS). Post-HSVE, and in some post Japanese encephalitis, there can be recurrent anti-NMDAR encephalitis in up to 20% of the children who typically present with new-onset choreoathetosis [45]. Similar overlaps may occur in the setting of ADS (MOG, NMOSD, MS) and anti-NMDAR encephalitis [45, 50]. The MRI patterns in post-HSVE-triggered AE are more commonly abnormal and have florid abnormalities including leukodystrophy-like phenotypes (Fig. 6) [50]. The mechanisms are possibly related to molecular mimicry, bystander activation, and epitope spreading [51].
Lastly, MOG encephalitis (MOGE) has emerged as one of the most common forms of pediatric AE and is within the expanding spectrum of MOG-IgG-associated disorders (MOGAD) [46, 52]. Interesting differences between adult and pediatric cohorts have emerged. Adult MOGE patterns include unilateral cortical involvement often limited to a particular lobe and considered a relatively benign phenotype [52]. Pediatric MOGE findings are on a spectrum and exhibit greater imaging variability including higher incidence of bilateral lesions, tumefactive demyelination (TD), subcortical and limbic involvement, leptomeningeal lesions, and can have diffusion changes (Fig. 7) [52]. TD can appear similar to phenotypes described with MS in children [53].
MOG-IgG-associated disorders (MOGAD) and tumefactive demyelination in MS. Case 1: axial FLAIR (A), axial post-contrast FLAIR (B), and post-contrast T1 (C), axial T2 and post-contrast FLAIR-cropped to orbits (D); Case 2: axial DWI (E, G), axial T2 (F), axial post-contrast T1 (H); Companion case: axial FLAIR (I), axial post-contrast T1 (J). Case 1: A 6-year-old, with low grade fever, headaches, and MOG encephalitis. There is unilateral localized cortical swelling and FLAIR hyperintensity involving the right mesial parietooccipital cortex (arrow, A), right hippocampus (not shown), cortical-subcortical (arrow, C), and leptomeningeal enhancement (arrow, B). Bilateral optic nerve head swelling and enhancement is noted (arrow, D). Case 2: A 10-year-old presenting with second episode of fever, somnolence, and seizures in a month. Mild patchy diffusion restriction in the right parietal cortex (oval outline) with no convincing FLAIR hyperintensity or enhancement (not shown). EEG showed focal right parietal focal slow waves, CSF showed significant pleocytosis. Recurrent acute presentation after 1 week shows tumefactive lesions with T2 hypointense rims, edema (arrows, F), and irregular ring-like enhancement (arrows, H). Companion case of MS (I, J) with tumefactive demyelination in MS with open rings of enhancement (arrow, J)
Genetic triggers of inflammation
Dysfunctions of diverse protein pathways can trigger brain inflammation, including proteins related to nuclear cargo trafficking, mitochondrial function, perforin regulation, and interferon signaling [54,55,56,57]. We discuss two prototypical pediatric inflammatory syndromes due to RANBP2 and perforin-dependent pathway mutations, and highlight mechanisms by which they compromise innate and adaptive immunity respectively.
Acute necrotising encephalopathy 1 (ANE1)—immune mechanisms and imaging
A prototypical example of a dysregulated interplay between infection, inflammation and genetics is acute necrotising encephalopathy 1 (ANE1). It is a febrile encephalopathy syndrome, often preceded by a non-specific infectious trigger, in which autosomal dominant mutations in Ran-binding protein 2 (RANBP2) or NUP358 render the CNS vulnerable to specific patterns of injury [58, 59].
The preceding infectious trigger, most commonly viral, need not have CNS affinity. These patients lack evidence of direct viral infiltration and an exaggerated immune response and cytokine storm is a common theme across the cases [36, 37]. Reminiscent of our prior discussions, systemic dyscytokinemia leads to an impaired BBB and initiation of an innate immune response. Much remains unknown of the further downstream CNS effects and how regionally specific patterns of brain injury emerge when co-existent RANBP2 mutations are present.
There have been recent developments in this understanding, specifically the role of RANBP2 as a small ubiquitin-related modifier (SUMO) pathway protein (Fig. 8). SUMO family proteins reversibly attach and stabilize target proteins (termed SUMOylation) to enable them to conduct vital cellular processes. In the context of CNS inflammation, RANBP2-mediated SUMOylation of specific proteins (Argonaute or AGO proteins) enables them to silence ANE-related cytokine mRNAs (IL-6 and TNFα) [60], STAT1 activity and type 1 interferon innate immune responses [61,62,63]. Failed silencing of IL-6 and other mRNAs may lead to persistent cytokine production and CNS injury (Fig. 8). These mechanisms are still being elaborated and may provide therapeutic targets in future [61, 64].
Nuclear pore complex and proposed role of RANBP2 SUMOylation in silencing inflammation. A Nuclear pore complex (NPC) is a large macromolecular structure embedded within the nuclear envelope and is formed by multiple proteins called nucleoporins (or NUPs). The proteins are organized in to substructures—including a central core (or inner ring) flanked on either side by cytoplasmic and nuclear rings. Nuclear pore complexes serve as conduits for import and export of cargo across the nuclear membrane. B Argonaute or AGO proteins are an extremely conserved family of proteins involved in messenger RNA (mRNA) silencing by enabling the attachment of a silencing RNA (in this context micro-RNA or miRNA-induced silencing complex, RISC) to the target mRNA (IL-6 mRNA). To do this, they require a stable attachment to the IL-6 mRNA. They achieve this stable attachment through RANBP2-mediated SUMOylation during their passage through the NPC. The SUMOylated AGO-IL-6 mRNA complex then attaches to miRNA-induced silencing complex, terminating IL-6 production. C It is unclear how a RANBP2 mutation affects this interaction but a failure of attachment of miRNA-induced silencing complex to an unstable AG0-IL-6 mRNA complex may enable persistent cytokine production and hypercytokinemia
MRI in ANE1 reveals characteristic imaging findings with multifocal bilateral involvement of thalami, brainstem, periventricular white matter, and cerebellum. Diffusion restriction, hemorrhagic foci, and enhancement from BBB breakdown are often co-existent (Supplementary Fig. 1). ANE1 has a non-genetic non-recurrent counterpart, termed ANE (without the numeric suffix) with some differences in regional lesion burden in comparison to ANE1 with the latter involving additional sites including claustrum and external capsule, medial temporal lobes, and spinal cord [58]. The differences in this distribution are still poorly understood.
There may be additional genetic drivers of ANE1 [65]. RANBP2-negative families with recurrent ANE are known, both from the original cohort and subsequently [58, 66] with these children being younger, more commonly boys and with worse outcomes in comparison to RANBP2-positive ANE1 [66]. While additional genetic loci still remain unknown, ANE-like imaging findings have been described with mutations in NUP214 (a functionally similar nucleoporin to RANBP2/NUP358) [54, 67], SCN1A, and carnitine palmitoyltransferase II (CPT2) polymorphisms [55, 68, 69].
Adaptive immunity, defects of perforin-dependent pathway, and primary HLH
Adaptive immunity consists of highly specialized pathways to remove the inciting trigger. Upon presentation of the target cell to cytotoxic T lymphocytes (CTLs), two highly specialized pathways may be engaged to eliminate infected cells – death-receptor pathway and perforin-dependent pathway (Supplementary Fig. 2) [70]. Perforin is a specialized pore-forming granzyme (a protease released by cytoplasmic granules) that is upregulated in deployed CTLs and mediates cell death through exocytosis and release in to the target cell. The series of steps from formation of these granzymes in T cells to the final degranulation and release at the target cell involve complex synergistically acting processes orchestrated through multiple genes (Fig. 9) [70, 71]. Genetic defects of the perforin pathway lead to primary hemophagocytic lymphohistiocytosis (HLH), characterized by uncontrolled immune T cell activation [71]. CNS involvement is characterized by disruptive BBB changes and T cell infiltration.
Role of specific proteins in target cell apoptosis and associated genetic defects in Perforin-dependent pathway. Abbreviations: LYST: lysosomal trafficking regulator, eSCRT: endosomal sorting complex required for transport, AP3B1: adaptor protein 3, CHS: Chediak–Higashi syndrome, HPS2: Hermansky – Pudlak syndrome type 2 adapted from [70, 71]
Despite the variability in the underlying genetic defect (Fig. 9), pediatric CNS HLH demonstrates regionally differing but recurring imaging patterns including multifocal cerebral and cerebellar lesions (Fig. 10), CLIPPERS-like presentation, and cerebellitis [57]. The diagnosis can be challenging in cases where presentations are CNS-isolated or precede systemic involvement and can have a relapsing phenotype. MRI patterns overlap with infections and ADS including NMOSD, MOG-AD, and tumefactive MS must be kept in mind in patients with multifocal lesions which remain poorly responsive to immune therapy [57].
CNS-isolated HLH at presentation in a 12-year-old child with PRF1 gene mutations. Fig 10a. Axial FLAIR (A1-6) and post-contrast T1 images (B1-2) showing multiple hyperintense lesions involving the cerebellum (solid white arrows) and brainstem (dashed white arrows) with areas of homogenous and nodular enhancement within. There is mass effect on the 4th ventricle, dilated temporal horns (black arrow, A6) and hydrocephalus. Fig 10b. CNS HLH at 5 months on treatment with progression and new lesion. Axial FLAIR (A1-2) and post-contrast T1 images (B1-2) showing interval decrease in the size and enhancement of the posterior fossa lesions (black arrows, A2, B2) with new FLAIR hyperintense lesion centered within the right parietal lobe with homogenous enhancement (dashed white arrow). Colour-coded CBV perfusion maps showing no increased perfusion within the new (white arrow) or prior lesions (black arrow)
COVID-19 neurological syndromes — an imaging perspective
Neurological injury in COVID-19 has been a matter of much speculation with competing theories proposing a direct neuropathic effect from neuroinvasion versus an immune-mediated brain inflammation. Much of the evidence has pointed to the latter [72, 73]. Despite the detection of viral inclusions in CNS [74] and SARS-CoV-2 RNA in CSF [73], autopsy studies have highlighted neuroimmune activation as a major cause for brain injury [75]. Studies of CSF cytokine profiles in patients with SARS-CoV-2-related encephalitis highlight an underlying cytokine-mediated CNS dysfunction akin to ICANS [72].
Consistent with the above observations, clinicoradiological peripheral and central nervous system syndromes in children with COVID-19 are predominantly of neuroinflammatory origin. The spectrum includes non-specific encephalopathy, ADEM, Guillain-Barré syndrome (Fig. 11), ANE, and AE (limbic encephalitis, cortical lesions, rhombencephalitis, cerebellitis) [73, 74, 76, 77].
Guillain-Barré Syndrome in COVID-19. Sagittal post-contrast T1 cervical (A, B) and lumbar spine (C), axial FLAIR (D), coronal T1 (E), and SWI (F). A 2-year-old previously healthy boy presenting with progressive lower and upper extremity weakness and hypotonia, ataxia, and febrile illness 2 weeks prior. Serology for COVID IgG was positive. CSF showed elevated protein. On MRI, there is multilevel enhancement of the exiting nerve roots in the cervical spine (A, B) and cauda equina (C). Asymmetrical patchy FLAIR hyperintensities in the peridentate regions (D) which persist after 1 year of follow-up. A branching T1 hyperintensity with blooming (arrow, F), favored to represent a small thrombosed vein
In pediatric cohorts, the most common parenchymal MRI pattern is that of single to multifocal lesions with an appearance reminiscent of ADEM with or without associated myelitis [74, 76, 78, 79]. There have been challenges in defining prevalence of antibody-positivity in this setting. Most systematically organized cohorts report antibody positivity as a rare finding [73, 74, 79,80,81], except for a single pediatric cohort study where the prevalence was 21% [76], likely due to differences in study design. Anti-MOG positivity rates in SARS-CoV2-associated ADEM have also been reported as rare when compared to other pediatric ADEM cohorts [79]. Antibody-positive acquired demyelinating syndromes (MOG-AD, NMOSD) and AE (NMDAR, MOGE) in SARS-CoV2 have presentations similar to established clinical phenotypes with these antibodies. Optic neuritis and multifocal ADEM-like lesions represent the most common imaging patterns related to anti-AQP4 and anti-MOG antibodies respectively [76].
On limited follow-up, relapses have not been reported in antibody positive ADS. Interestingly, studies assessing pediatric ADEM SARS-CoV2 cohorts demonstrated better steroid responsiveness and outcomes [76] when compared to adult counterparts which were associated with severe infections, hemorrhage, high morbidity, and mortality [79].
Pediatric SARS-CoV2-related neuroinflammatory disease can also occur in the setting of multisystem inflammatory syndrome in children (MIS-C), alternatively termed pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) [82].The reported prevalence of neurological abnormalities is low (12–20%) [77, 82, 83] and may be associated with higher systemic inflammatory markers [82]. Neuroinflammation in this setting is characterized by encephalopathy and broad neurological symptoms, often with involvement of the peripheral nervous system. Work-up demonstrates increased systemic inflammatory markers, a frequently normal CSF and imaging findings different from acquired demyelinating syndromes [76, 77, 82, 83]. MRI abnormalities are infrequent, although when present, central splenial T2-hyperintensity with restricted diffusion is described, reported at a variable prevalence of 11–64% [74, 76, 82, 83]. Signal changes can extend to the rest of the corpus callosum and adjacent white matter and show complete reversibility. In most cohorts of MIS-C, this is reported as an isolated finding occurring independently of seizures, except in a single study where co-existent ADEM-like lesions were present in a similar prevalence [74]. Evidence from CSF and systemic cytokine profiles, and similarities to cytotoxic lesions of corpus callosum (CLOCCs) suggests intramyelinic edema from excitotoxic injury as the likely causal mechanism [83].
In smaller numbers, vascular phenomena have also been reported in MIS-C- including infarcts, parenchymal hemorrhage, cerebral venous thrombosis, and microhemorrhages from thrombotic microangiopathy [74, 77, 82]. Vascular manifestations are an important subset of CNS injury in adult SARS-CoV2 cohorts. These are well described in children as well, but overall burden is likely lower [74, 77].
Conclusion
We elaborate how innate and adaptive immune mechanisms play a role in pediatric CNS inflammation. Inflammatory, infectious, and genetic disorders may utilize common immune mechanisms to produce overlapping yet distinct patterns of CNS injury. These imaging patterns are closely linked to shared immune drivers. We corroborate this through an imaging review and discuss the recent updates in our understanding of these mechanisms.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials. The illustrations were Created with BioRender.com.
Abbreviations
- BBB:
-
Blood–brain barrier
- APC:
-
Antigen presenting cell
- TLR:
-
Toll-like receptor
- PRES:
-
Posterior reversible encephalopathy syndrome
- ISF:
-
Interstitial fluid
- MLV:
-
Meningeal lymphatic vessel
- FIRES:
-
Febrile infection-related epilepsy syndrome
- ICANS:
-
Immune effector cell-associated neurotoxicity syndrome
- HPeV3:
-
Human Parechovirus 3
- HSVE:
-
HSV encephalitis
- ADS:
-
Acquired demyelinating syndromes
- MOGAD:
-
MOG-IgG-associated disorders
- NMOSD:
-
Neuromyelitis optica spectrum disorder
- Anti-NMDAR:
-
Anti-N-methyl-D-aspartate receptor
- AE:
-
Autoimmune encephalitis
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- MS:
-
Multiple sclerosis
References
Galea I, Bechmann I, Perry VH (2007) What is immune privilege (not)? Trends Immunol 28:12–18. https://doi.org/10.1016/j.it.2006.11.004
Meyer C, Martin-Blondel G, Liblau RS (2017) Endothelial cells and lymphatics at the interface between the immune and central nervous systems: implications for multiple sclerosis. Curr Opin Neurol 30:222–230. https://doi.org/10.1097/WCO.0000000000000454
Ratnam NM, Gilbert MR, Giles AJ (2019) Immunotherapy in CNS cancers: the role of immune cell trafficking. Neuro Oncol 21:37–46. https://doi.org/10.1093/neuonc/noy084
Galea I (2021) The blood–brain barrier in systemic infection and inflammation. Cell Mol Immunol 18:2489–2501. https://doi.org/10.1038/s41423-021-00757-x
Varatharaj A, Galea I (2017) The blood-brain barrier in systemic inflammation. Brain Behav Immun 60:1–12. https://doi.org/10.1016/j.bbi.2016.03.010
Balasa R, Barcutean L, Mosora O, Manu D (2021) Reviewing the significance of blood–brain barrier disruption in multiple sclerosis pathology and treatment. Int J Mol Sci 22:8370. https://doi.org/10.3390/ijms22168370
Louveau A, Smirnov I, Keyes TJ et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341. https://doi.org/10.1038/nature14432
Aspelund A, Antila S, Proulx ST et al (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212:991–999. https://doi.org/10.1084/jem.20142290
Louveau A, Herz J, Alme MN et al (2018) CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci 21:1380–1391. https://doi.org/10.1038/s41593-018-0227-9
Da Mesquita S, Fu Z, Kipnis J (2018) The Meningeal lymphatic system: a new player in neurophysiology. Neuron 100:375–388. https://doi.org/10.1016/j.neuron.2018.09.022
Sapkota D, Florian C, Doherty BM et al (2022) Aqp4 stop codon readthrough facilitates amyloid-β clearance from the brain. Brain 145:2982–2990. https://doi.org/10.1093/brain/awac199
Carlstrom LP, Eltanahy A, Perry A et al (2022) A clinical primer for the glymphatic system. Brain 145:843–857. https://doi.org/10.1093/brain/awab428
Traka M, Podojil JR, McCarthy DP et al (2016) Oligodendrocyte death results in immune-mediated CNS demyelination. Nat Neurosci 19:65–74. https://doi.org/10.1038/nn.4193
van Zwam M, Huizinga R, Heijmans N et al (2009) Surgical excision of CNS-draining lymph nodes reduces relapse severity in chronic-relapsing experimental autoimmune encephalomyelitis. J Pathol 217:543–551. https://doi.org/10.1002/path.2476
Absinta M, Ha S-K, Nair G et al (2017) Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. eLife 6:e29738. https://doi.org/10.7554/eLife.29738
Li X, Qi L, Yang D et al (2022) Meningeal lymphatic vessels mediate neurotropic viral drainage from the central nervous system. Nat Neurosci 25:577–587. https://doi.org/10.1038/s41593-022-01063-z
Da Mesquita S, Louveau A, Vaccari A et al (2018) Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560:185–191. https://doi.org/10.1038/s41586-018-0368-8
Kigerl KA, de Rivero Vaccari JP, Dietrich WD et al (2014) Pattern recognition receptors and central nervous system repair. Exp Neurol 258:5–16. https://doi.org/10.1016/j.expneurol.2014.01.001
Shoshkes Reiss C (2008) Neurotropic viral infections. Cambridge University Press, Cambridge
Nguyen MD, Julien J-P, Rivest S (2002) Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci 3:216–227. https://doi.org/10.1038/nrn752
Mørk N, Kofod-Olsen E, Sørensen KB et al (2015) Mutations in the TLR3 signaling pathway and beyond in adult patients with herpes simplex encephalitis. Genes Immun 16:552–566. https://doi.org/10.1038/gene.2015.46
Bach J-F (2018) The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol 18:105–120. https://doi.org/10.1038/nri.2017.111
Cepon-Robins TJ, Gildner TE (2020) Old friends meet a new foe: a potential role for immune-priming parasites in mitigating COVID-19 morbidity and mortality. Evol Med Publ Health 2020:234–248. https://doi.org/10.1093/emph/eoaa037
Moltoni G, D’Arco F, Pasquini L et al (2020) Non-congenital viral infections of the central nervous system: from the immunocompetent to the immunocompromised child. Pediatr Radiol 50:1757–1767. https://doi.org/10.1007/s00247-020-04746-6
Verboon-Maciolek MA, Groenendaal F, Hahn CD et al (2008) Human parechovirus causes encephalitis with white matter injury in neonates. Ann Neurol 64:266–273. https://doi.org/10.1002/ana.21445
de Vries LS (2019) Viral Infections and the neonatal brain. Semin Pediatr Neurol 32:100769. https://doi.org/10.1016/j.spen.2019.08.005
Sarma A, Hanzlik E, Krishnasarma R et al (2019) Human Parechovirus meningoencephalitis: neuroimaging in the era of polymerase chain reaction–based testing. AJNR Am J Neuroradiol 40:1418–1421. https://doi.org/10.3174/ajnr.A6118
Khamkar A, Suryawanshi P, Pote PD, Jose GE (2022) Neonatal encephalitis and white matter injury in an early neonate: cause or association with COVID-19 infection? J Neonatol 09732179221087359. https://doi.org/10.1177/09732179221087359
Volpe JJ (2008) Neonatal encephalitis and white matter injury: more than just inflammation? Ann Neurol 64:232–236. https://doi.org/10.1002/ana.21466
Bissel SJ, Auer RN, Chiang C-H et al (2015) Human Parechovirus 3 meningitis and fatal leukoencephalopathy. J Neuropathol Exp Neurol 74:767–777. https://doi.org/10.1097/NEN.0000000000000215
Lane LM, McDermott MB, O’Connor P et al (2021) Multicystic encephalomalacia: the neuropathology of systemic neonatal parechovirus infection. Pediatr Dev Pathol 24:460–466. https://doi.org/10.1177/10935266211001645
Arrigoni F, Parazzini C, Righini A et al (2011) Deep medullary vein involvement in neonates with brain damage: an MR imaging study. AJNR Am J Neuroradiol 32:2030–2036. https://doi.org/10.3174/ajnr.A2687
Sahu PK, Hoffmann A, Majhi M et al (2021) Brain magnetic resonance imaging reveals different courses of disease in pediatric and adult cerebral malaria. Clin Infect Dis 73:e2387–e2396. https://doi.org/10.1093/cid/ciaa1647
Potchen MJ, Kampondeni SD, Seydel KB et al (2012) Acute brain MRI findings in 120 Malawian children with cerebral malaria: new insights into an ancient disease. AJNR Am J Neuroradiol 33:1740–1746. https://doi.org/10.3174/ajnr.A3035
Moghaddam SM, Birbeck GL, Taylor TE et al (2019) Diffusion-weighted MR Imaging in a prospective cohort of children with cerebral malaria offers insights into pathophysiology and prognosis. AJNR Am J Neuroradiol 40:1575–1580. https://doi.org/10.3174/ajnr.A6159
Rasalkar DD, Paunipagar BK, Sanghvi D et al (2011) Magnetic resonance imaging in cerebral malaria: a report of four cases. Br J Radiol 84:380–385. https://doi.org/10.1259/bjr/85759874
Dorovini-Zis K, Schmidt K, Huynh H et al (2011) The neuropathology of fatal cerebral malaria in malawian children. Am J Pathol 178:2146–2158. https://doi.org/10.1016/j.ajpath.2011.01.016
Muccioli L, Pensato U, Vito LD et al (2022) Teaching NeuroImage: claustrum sign in febrile infection–related epilepsy syndrome. Neurology 98:e1090–e1091. https://doi.org/10.1212/WNL.0000000000013261
Sperner J, Sander B, Lau S et al (1996) Severe transitory encephalopathy with reversible lesions of the claustrum. Pediatr Radiol 26:769–771. https://doi.org/10.1007/BF01396197
Kimura S, Nezu A, Osaka H, Saito K (1994) Symmetrical external capsule lesions in a patient with herpes simplex encephalitis. Neuropediatrics 25:162–164. https://doi.org/10.1055/s-2008-1073016
Meletti S, Slonkova J, Mareckova I et al (2015) Claustrum damage and refractory status epilepticus following febrile illness. Neurology 85:1224–1232. https://doi.org/10.1212/WNL.0000000000001996
Steriade C, Tang-Wai DF, Krings T, Wennberg R (2017) Claustrum hyperintensities: a potential clue to autoimmune epilepsy. Epilepsia Open 2:476. https://doi.org/10.1002/epi4.12077
Saito Y, Maegaki Y, Okamoto R et al (2007) Acute encephalitis with refractory, repetitive partial seizures: case reports of this unusual post-encephalitic epilepsy. Brain Develop 29:147–156. https://doi.org/10.1016/j.braindev.2006.08.005
Pensato U, Muccioli L, Cani I et al (2021) Brain dysfunction in COVID-19 and CAR-T therapy: cytokine storm-associated encephalopathy. Ann Clin Transl Neurol 8:968–979. https://doi.org/10.1002/acn3.51348
Graus F, Titulaer MJ, Balu R et al (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15:391–404. https://doi.org/10.1016/S1474-4422(15)00401-9
Cellucci T, Mater HV, Graus F et al (2020) Clinical approach to the diagnosis of autoimmune encephalitis in the pediatric patient. Neurol - Neuroimmunol Neuroinflammation 7. https://doi.org/10.1212/NXI.0000000000000663
Hardy D (2022) Autoimmune encephalitis in children. Pediatr Neurol 132:56–66. https://doi.org/10.1016/j.pediatrneurol.2022.05.004
Saket RR, Geschwind MD, Josephson SA et al (2011) Autoimmune-mediated encephalopathy: classification, evaluation, and MR Imaging Patterns of Disease. Neurographics 1:2–16. https://doi.org/10.3174/ng.1110001
Renard D, Nerrant E, Lechiche C (2015) DWI and FLAIR imaging in herpes simplex encephalitis: a comparative and topographical analysis. J Neurol 262:2101–2105. https://doi.org/10.1007/s00415-015-7818-0
Hacohen Y, Rossor T, Mankad K et al (2018) “Leukodystrophy-like” phenotype in children with myelin oligodendrocyte glycoprotein antibody-associated disease. Dev Med Child Neurol 60:417–423. https://doi.org/10.1111/dmcn.13649
Piquet AL, Clardy SL (2018) Infection, Immunodeficiency, and inflammatory diseases in autoimmune neurology. Semin Neurol 38:379–391. https://doi.org/10.1055/s-0038-1660820
Tenembaum SN (2021) Pediatric demyelinating disease and anti-MOG antibody. Clin Exp Neuroimmunol 12:7–21. https://doi.org/10.1111/cen3.12627
Sánchez P, Chan F, Hardy TA (2021) Tumefactive demyelination: updated perspectives on diagnosis and management. Expert Rev Neurother 21:1005–1017. https://doi.org/10.1080/14737175.2021.1971077
Benarroch EE (2019) Nucleocytoplasmic transport: Mechanisms and involvement in neurodegenerative disease. Neurology 92:757–764. https://doi.org/10.1212/WNL.0000000000007305
Ohashi E, Hayakawa I, Murofushi Y et al (2021) Recurrent acute necrotizing encephalopathy in a boy with RANBP2 mutation and thermolabile CPT2 variant: The first case of ANE1 in Japan. Brain Dev 43:873–878. https://doi.org/10.1016/j.braindev.2021.04.009
La Piana R, Uggetti C, Roncarolo F et al (2016) Neuroradiologic patterns and novel imaging findings in Aicardi-Goutières syndrome. Neurology 86:28–35. https://doi.org/10.1212/WNL.0000000000002228
Malik P, Antonini L, Mannam P et al (2021) MRI patterns in pediatric cns hemophagocytic lymphohistiocytosis. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A7292
Neilson DE, Adams MD, Orr CMD et al (2009) Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am J Hum Genet 84:44–51. https://doi.org/10.1016/j.ajhg.2008.12.009
Levine JM, Ahsan N, Ho E, Santoro JD (2020) Genetic acute necrotizing encephalopathy associated with RANBP2: clinical and therapeutic implications in pediatrics. Mult Scler Relat Disord 43:102194. https://doi.org/10.1016/j.msard.2020.102194
Hutvagner G, Simard MJ (2008) Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol 9:22–32. https://doi.org/10.1038/nrm2321
Palazzo AF, Joseph J, Lim M, Thakur K (2022) Workshop on RanBP2/Nup358 and acute necrotizing encephalopathy. Nucleus. https://doi.org/10.1080/19491034.2022.2069071
Shen Q, Wang YE, Truong M et al (2021) RanBP2/Nup358 enhances miRNA activity by sumoylating Argonautes. PLoS Genet 17:e1009378. https://doi.org/10.1371/journal.pgen.1009378
Sahoo MR, Gaikwad S, Khuperkar D et al (2017) Nup358 binds to AGO proteins through its SUMO-interacting motifs and promotes the association of target mRNA with miRISC. EMBO Rep 18:241–263. https://doi.org/10.15252/embr.201642386
Shen Q, Wang YE, Palazzo AF (2021) Crosstalk between nucleocytoplasmic trafficking and the innate immune response to viral infection. J Biol Chem 297:100856. https://doi.org/10.1016/j.jbc.2021.100856
Neilson DE (2010) The interplay of infection and genetics in acute necrotizing encephalopathy. Curr Opin Pediatr 22:751–757. https://doi.org/10.1097/MOP.0b013e3283402bfe
Nishimura N, Higuchi Y, Kimura N et al (2016) Familial acute necrotizing encephalopathy without RANBP2 mutation: poor outcome. Pediatr Int 58:1215–1218. https://doi.org/10.1111/ped.13119
Fichtman B, Harel T, Biran N et al (2019) Pathogenic variants in NUP214 cause “plugged” nuclear pore channels and acute febrile encephalopathy. Am J Hum Genet 105:48–64. https://doi.org/10.1016/j.ajhg.2019.05.003
Kubota M, Chida J, Hoshino H et al (2012) Thermolabile CPT II variants and low blood ATP levels are closely related to severity of acute encephalopathy in Japanese children. Brain Dev 34:20–27. https://doi.org/10.1016/j.braindev.2010.12.012
Saitoh M, Shinohara M, Hoshino H et al (2012) Mutations of the SCN1A gene in acute encephalopathy. Epilepsia 53:558–564. https://doi.org/10.1111/j.1528-1167.2011.03402.x
Voskoboinik I, Smyth MJ, Trapani JA (2006) Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol 6:940–952. https://doi.org/10.1038/nri1983
de Saint BG, Ménasché G, Fischer A (2010) Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat Rev Immunol 10:568–579. https://doi.org/10.1038/nri2803
Pilotto A, Masciocchi S, Volonghi I et al (2021) Severe Acute respiratory syndrome coronavirus 2 (SARS-CoV-2) encephalitis is a cytokine release syndrome: evidences from cerebrospinal fluid analyses. Clin Infect Dis 73:e3019–e3026. https://doi.org/10.1093/cid/ciaa1933
Zamani R, Pouremamali R, Rezaei N (2022) Central neuroinflammation in Covid-19: a systematic review of 182 cases with encephalitis, acute disseminated encephalomyelitis, and necrotizing encephalopathies. Rev Neurosci 33:397–412. https://doi.org/10.1515/revneuro-2021-0082
Lindan CE, Mankad K, Ram D et al (2021) Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health 5:167–177. https://doi.org/10.1016/S2352-4642(20)30362-X
Matschke J, Lütgehetmann M, Hagel C et al (2020) Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 19:919–929. https://doi.org/10.1016/S1474-4422(20)30308-2
Aubart M, Roux C-J, Durrleman C et al (2022) Neuroinflammatory Disease following severe acute respiratory syndrome coronavirus 2 infection in children. J Pediatr S0022–3476(22):00426–00427. https://doi.org/10.1016/j.jpeds.2022.05.018
LaRovere KL, Riggs BJ, Poussaint TY et al (2021) Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol 78:536–547. https://doi.org/10.1001/jamaneurol.2021.0504
Signa S, Brolatti N, Trincianti C et al (2022) Pediatric SARS-CoV2–related diplopia and mesencephalic abnormalities. Neurol: Clin Pract. https://doi.org/10.1212/CPJ.0000000000200076
Manzano GS, McEntire CRS, Martinez-Lage M et al (2021) Acute disseminated encephalomyelitis and acute hemorrhagic leukoencephalitis following COVID-19: systematic review and meta-synthesis. Neurol Neuroimmunol Neuroinflamm 8:e1080. https://doi.org/10.1212/NXI.0000000000001080
Paterson RW, Brown RL, Benjamin L et al (2020) The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain 143:3104–3120. https://doi.org/10.1093/brain/awaa240
Sanchez CV, Theel E, Binnicker M et al (2021) Autoimmune encephalitis after SARS-CoV-2 infection: case frequency, findings, and outcomes. Neurology 97:e2262–e2268. https://doi.org/10.1212/WNL.0000000000012931
Sa M, Mirza L, Carter M et al (2021) Systemic inflammation is associated with neurologic involvement in pediatric inflammatory multisystem syndrome associated with SARS-CoV-2. Neurol - Neuroimmunol Neuroinflammation 8. https://doi.org/10.1212/NXI.0000000000000999
Abdel-Mannan O, Eyre M, Löbel U et al (2020) Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.2687
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/competing interests (include appropriate disclosures)
The authors declare that they have no known competing financial or personal relationships that could be viewed as influencing the work reported in this paper.
Ethics approval (include appropriate approvals or waivers)
The authors declare that all procedures performed were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent (include appropriate statements)
The authors declare that this report does not contain any personal information or data that could lead to the identification of the patient(s) and/or volunteers and consent was waived.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malik, P., Shroff, M. Infection and inflammation: radiological insights into patterns of pediatric immune-mediated CNS injury. Neuroradiology 65, 425–439 (2023). https://doi.org/10.1007/s00234-022-03100-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03100-x