Abstract
Purpose
The mutations of the glucocerebrosidase (GBA) gene are the greatest genetic risk factor for Parkinson’s disease (PD). The mechanism underlying the association between GBA mutations and PD has not been fully elucidated.
Methods
Using resting-state functional magnetic resonance imaging and graph theory analysis to investigate the disrupted topological organization in PD patients with GBA mutation (GBA-PD). Eleven GBA-PD patients, 11 noncarriers with PD, and 18 healthy controls (HCs) with a similar age and sex distribution were recruited. Individual whole-brain functional connectome was constructed, and the global and nodal topological disruptions were calculated among groups. Partial correlation analyses between the clinical features of patients with PD and topological alterations were performed.
Results
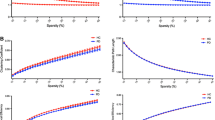
The GBA-PD group showed prominently decreased characteristic path length (Lp) and increased global efficiency (Eg) compared to HCs at the global level; a significantly increased nodal betweenness centrality in the medial prefrontal cortex (mPFC) and precuneus within the default mode network, and precentral gyrus within the sensorimotor network, while a significantly decreased betweenness centrality in nodes within the cingulo-opercular network compared to the noncarrier group at the regional level. The altered nodal betweenness centrality of mPFC was positively correlated with fatigue severity scale scores in all patients with PD.
Conclusion
The preliminary pilot study found that GBA-PD patients had a higher functional integration at the global level. The nodal result of the mPFC is congruent with the potential fatigue pathology in PD and is suggestive of a profound effect of GBA mutations on the clinical fatigue in patients with PD.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Bloem BR, Okun MS, Klein C (2021) Parkinson’s disease. Lancet 397:2284–2303. https://doi.org/10.1016/S0140-6736(21)00218-X
Sidransky E, Nalls MA, Aasly JO et al (2009) Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med 361:1651–1661. https://doi.org/10.1056/NEJMoa0901281
Balestrino R, Schapira AHV (2018) Glucocerebrosidase and Parkinson disease: molecular, clinical, and therapeutic implications. Neuroscientist 24:540–559. https://doi.org/10.1177/1073858417748875
Blandini F, Cilia R, Cerri S et al (2019) Glucocerebrosidase mutations and synucleinopathies: toward a model of precision medicine. Mov Disord 34:9–21. https://doi.org/10.1002/mds.27583
Saeed U, Lang AE, Masellis M (2020) Neuroimaging advances in Parkinson’s disease and atypical Parkinsonian syndromes. Front Neurol 11:572976. https://doi.org/10.3389/fneur.2020.572976
Filippi M, Balestrino R, Basaia S, Agosta F (2022) Neuroimaging in glucocerebrosidase-associated Parkinsonism: a systematic review. Mov Disord 37:1375–1393. https://doi.org/10.1002/mds.29047
Leocadi M, Canu E, Donzuso G et al (2022) Longitudinal clinical, cognitive, and neuroanatomical changes over 5 years in GBA-positive Parkinson’s disease patients. J Neurol 269:1485–1500. https://doi.org/10.1007/s00415-021-10713-4
Agosta F, Kostic VS, Davidovic K et al (2013) White matter abnormalities in Parkinson’s disease patients with glucocerebrosidase gene mutations. Mov Disord 28:772–778. https://doi.org/10.1002/mds.25397
Thaler A (2018) Structural and functional MRI in familial Parkinson’s disease. Int Rev Neurobiol 142:261–287. https://doi.org/10.1016/bs.irn.2018.09.005
Greuel A, Trezzi JP, Glaab E et al (2020) GBA Variants in Parkinson’s disease: clinical, metabolomic, and multimodal neuroimaging phenotypes. Mov Disord 35:2201–2210. https://doi.org/10.1002/mds.28225
Luo CY, Guo XY, Song W et al (2015) Functional connectome assessed using graph theory in drug-naive Parkinson’s disease. J Neurol 262:1557–1567. https://doi.org/10.1007/s00415-015-7750-3
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Emre M, Aarsland D, Brown R et al (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22:1689–1707. https://doi.org/10.1002/mds.21507 (quiz 1837)
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653. https://doi.org/10.1002/mds.23429
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. https://doi.org/10.1016/j.neuroimage.2011.10.018
Hou Y, Wei Q, Ou R, Yang J, Gong Q, Shang H (2020) Impaired topographic organization in Parkinson’s disease with mild cognitive impairment. J Neurol Sci 414:116861. https://doi.org/10.1016/j.jns.2020.116861
Medaglia JD (2017) Graph Theoretic analysis of resting state functional MR imaging. Neuroimaging Clin N Am 27:593–607. https://doi.org/10.1016/j.nic.2017.06.008
Dosenbach NU, Nardos B, Cohen AL et al (2010) Prediction of individual brain maturity using fMRI. Science 329:1358–1361. https://doi.org/10.1126/science.1194144
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069. https://doi.org/10.1016/j.neuroimage.2009.10.003
Sreenivasan K, Mishra V, Bird C et al (2019) Altered functional network topology correlates with clinical measures in very early-stage, drug-naive Parkinson’s disease. Parkinsonism Relat Disord 62:3–9. https://doi.org/10.1016/j.parkreldis.2019.02.001
Suo X, Lei D, Li N et al (2017) Functional brain connectome and its relation to Hoehn and Yahr stage in Parkinson disease. Radiology 285:904–913. https://doi.org/10.1148/radiol.2017162929
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211. https://doi.org/10.1016/s0197-4580(02)00065-9
Meppelink AM, de Jong BM, Renken R, Leenders KL, Cornelissen FW, van Laar T (2009) Impaired visual processing preceding image recognition in Parkinson’s disease patients with visual hallucinations. Brain 132:2980–2993. https://doi.org/10.1093/brain/awp223
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682. https://doi.org/10.1073/pnas.98.2.676
Xu P, Chen A, Li Y, Xing X, Lu H (2019) Medial prefrontal cortex in neurological diseases. Physiol Genomics 51:432–442. https://doi.org/10.1152/physiolgenomics.00006.2019
Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129:564–583. https://doi.org/10.1093/brain/awl004
Ramos BP, Arnsten AF (2007) Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther 113:523–536. https://doi.org/10.1016/j.pharmthera.2006.11.006
Friedman JH, Brown RG, Comella C et al (2007) Fatigue in Parkinson’s disease: a review. Mov Disord 22:297–308. https://doi.org/10.1002/mds.21240
Chaudhuri A, Behan PO (2004) Fatigue in neurological disorders. Lancet 363:978–988. https://doi.org/10.1016/S0140-6736(04)15794-2
Roelcke U, Kappos L, Lechner-Scott J et al (1997) Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology 48:1566–1571. https://doi.org/10.1212/wnl.48.6.1566
Strotzer QD, Kohl Z, Anthofer JM et al (2022) Structural connectivity patterns of side effects induced by subthalamic deep brain stimulation for Parkinson’s disease. Brain Connect 12:374–384. https://doi.org/10.1089/brain.2021.0051
Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:655–667. https://doi.org/10.1007/s00429-010-0262-0
Wolters AF, van de Weijer SCF, Leentjens AFG, Duits AA, Jacobs HIL, Kuijf ML (2019) Resting-state fMRI in Parkinson’s disease patients with cognitive impairment: a meta-analysis. Parkinsonism Relat Disord 62:16–27. https://doi.org/10.1016/j.parkreldis.2018.12.016
Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ (2010) Fatigue in Parkinson’s disease is linked to striatal and limbic serotonergic dysfunction. Brain 133:3434–3443. https://doi.org/10.1093/brain/awq268
Wang J, Wang L, Zang Y et al (2009) Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum Brain Mapp 30:1511–1523. https://doi.org/10.1002/hbm.20623
Acknowledgements
The authors thank the patients and their families for their participation in the study.
Funding
The present study was supported by the National Key Research and Development Program of China (Grant No. 2021YFC2501203 to FHS); and the Sichuan Science and Technology Program (Grant No. 2022ZDZX0023 to FHS).
Author information
Authors and Affiliations
Contributions
Yanbing Hou: conception and design of the study, statistical analysis, interpretation of data, and drafting of manuscript
Fei Feng: data collection and statistical analysis
Lingyu Zhang: data collection and statistical analysis
Ruwei Ou: data collection
Junyu Lin: data collection
Qiyong Gong: study design, analysis and interpretation, and revision of the manuscript
Huifang Shang: study design, analysis and interpretation, and revision of the manuscript
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Approval was received from the West China Hospital of Sichuan University Clinical trials and Biomedical ethics committee.
Consent to participate
Written informed consent was obtained from all participants in the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hou, Y., Feng, F., Zhang, L. et al. Disrupted topological organization of resting-state functional brain networks in Parkinson’s disease patients with glucocerebrosidase gene mutations. Neuroradiology 65, 361–370 (2023). https://doi.org/10.1007/s00234-022-03067-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03067-9