Abstract
Purpose
Bayesian estimation with advanced noise reduction (BEANR) in CT perfusion (CTP) could deliver more reliable cerebral blood flow (CBF) measurements than the commonly used reformulated singular value decomposition (rSVD). We compared the efficacy of CBF measurement by CTP using BEANR and rSVD, evaluating both relative to N-isopropyl-p-[(123) I]- iodoamphetamine (123I-IMP) single-photon emission computed tomography (SPECT) as a reference standard, in patients with cerebrovascular disease.
Methods
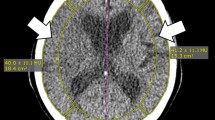
Thirty-one patients with suspected cerebrovascular disease underwent both CTP on a 320 detector-row CT system and SPECT. We applied rSVD and BEANR in the ischemic and contralateral regions to create CBF maps and calculate CBF ratios from the ischemic side to the healthy contralateral side (CBF index). The analysis involved comparing the CBF index between CTP methods and SPECT using Pearson’s correlation and limits of agreement determined with Bland–Altman analyses, before comparing the mean difference in the CBF index between each CTP method and SPECT using the Wilcoxon matched pairs signed-rank test.
Results
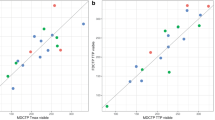
The CBF indices of BEANR and 123I-IMP SPECT were significantly and positively correlated (r = 0.55, p < 0.0001), but there was no significant correlation between the rSVD method and SPECT (r = 0.15, p > 0.05). BEANR produced smaller limits of agreement for CBF than rSVD. The mean difference in the CBF index between BEANR and SPECT differed significantly from that between rSVD and SPECT (p < 0.001).
Conclusions
BEANR has a better potential utility for CBF measurement in CTP than rSVD compared to SPECT in patients with cerebrovascular disease.







Similar content being viewed by others
Data availability
Data generated or analyzed during the study are available from the corresponding author by request.
Code availability
Software application: Vitrea workstation (Brain Perfusion 4D, Vital Images, Minnetonka, MN, USA).
References
Klotz E, König M (1999) Perfusion measurements of the brain: using dynamic CT for the quantitative assessment of cerebral ischemia in acute stroke. Eur J Radiol 30:170–184. https://doi.org/10.1016/s0720-048x(99)00009-1
Mayer TE, Hamann GF, Baranczyk J et al (2000) Dynamic CT perfusion imaging of acute stroke. AJNR Am J Neuroradiol 21:1441–1449
Lev MH, Segal AZ, Farkas J et al (2001) Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome. Stroke 32:2021–2028. https://doi.org/10.1161/hs0901.095680
Sakai F, Nakazawa K, Tazaki Y et al (1985) Regional cerebral blood volume and hematocrit measured in normal human volunteers by single-photon emission computed tomography. J Cereb Blood Flow Metab 5:207–213. https://doi.org/10.1038/jcbfm.1985.27
Murayama K, Toyama H, Hayakawa M et al (2014) Voxel-based correlation between whole-brain CT perfusion with 320-row area detector CT and iodine 123 iodoamphetamine brain perfusion spect in patients with cerebrovascular disease. J Comput Assist Tomogr 38:639–646. https://doi.org/10.1097/rct.0000000000000110
Siebert E, Bohner G, Dewey M et al (2009) 320-slice CT neuroimaging: initial clinical experience and image quality evaluation. Br J Radiol 82:561–570. https://doi.org/10.1259/bjr/27721218
Berkhemer OA, Fransen PS, Beumer D et al (2015) A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372:11–20. https://doi.org/10.1056/NEJMoa1411587
Campbell BC, Mitchell PJ, Kleinig TJ et al (2015) Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372:1009–1018. https://doi.org/10.1056/NEJMoa1414792
Goyal M, Demchuk AM, Menon BK et al (2015) Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372:1019–1030. https://doi.org/10.1056/NEJMoa1414905
Wu O, Østergaard L, Weisskoff RM, Benner T, Rosen BR, Sorensen AG (2003) Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med 50:164–174. https://doi.org/10.1002/mrm.10522
Fieselmann A, Kowarschik M, Ganguly A, Hornegger J, Fahrig R (2011) Deconvolution-based CT and MR brain perfusion measurement: theoretical model revisited and practical implementation details. Int J Biomed Imaging 2011:467563. https://doi.org/10.1155/2011/467563
Konstas AA, Goldmakher GV, Lee TY, Lev MH (2009) Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 2: technical implementations. AJNR Am J Neuroradiol 30:885–892. https://doi.org/10.3174/ajnr.A1492
Murayama K, Katada K, Hayakawa M, Toyama H (2017) Shortened mean transit time in CT perfusion with singular value decomposition analysis in acute cerebral infarction: quantitative evaluation and comparison with various CT perfusion parameters. J Comput Assist Tomogr 41:173–180. https://doi.org/10.1097/rct.0000000000000498
Mouridsen K, Friston K, Hjort N, Gyldensted L, Ostergaard L, Kiebel S (2006) Bayesian estimation of cerebral perfusion using a physiological model of microvasculature. Neuroimage 33:570–579. https://doi.org/10.1016/j.neuroimage.2006.06.015
Boutelier T, Kudo K, Pautot F, Sasaki M (2012) Bayesian hemodynamic parameter estimation by bolus tracking perfusion weighted imaging. IEEE Trans Med Imaging 31:1381–1395. https://doi.org/10.1109/tmi.2012.2189890
Nael K, Mossadeghi B, Boutelier T et al (2015) Bayesian estimation of cerebral perfusion using reduced-contrast-dose dynamic susceptibility contrast perfusion at 3T. AJNR Am J Neuroradiol 36:710–718. https://doi.org/10.3174/ajnr.A4184
Nael K, Tadayon E, Wheelwright D et al (2019) Defining ischemic core in acute ischemic stroke using CT perfusion: a multiparametric bayesian-based model. AJNR Am J Neuroradiol 40:1491–1497. https://doi.org/10.3174/ajnr.A6170
Smith MR, Lu H, Trochet S (2004) R Frayne (2004) Removing the effect of SVD algorithmic artifacts present in quantitative MR perfusion studies. Magn Reson Med. 51(3):631–4. https://doi.org/10.1002/mrm.20006
Murayama K, Katada K, Nakane M et al (2009) Whole-brain perfusion CT performed with a prototype 256-detector-row CT system: initial experience. Radiology 250:202–211. https://doi.org/10.1148/radiol.2501071809
Ichihara T, Ogawa K, Motomura N, Kubo A, Hashimoto S (1993) Compton scatter compensation using the triple-energy window method for single- and dual-isotope SPECT. J Nucl Med 34:2216–2221
Ishii K, Hanaoka K, Okada M et al (2012) Impact of CT attenuation correction by SPECT/CT in brain perfusion images. Ann Nucl Med 26:241–247. https://doi.org/10.1007/s12149-011-0567-y
Inui Y, Ichihara T, Uno M et al (2018) CT-based attenuation correction and resolution compensation for I-123 IMP brain SPECT normal database: a multicenter phantom study. Ann Nucl Med 32:311–318. https://doi.org/10.1007/s12149-018-1248-x
Kudo K, Terae S, Katoh C et al (2003) Quantitative cerebral blood flow measurement with dynamic perfusion CT using the vascular-pixel elimination method: comparison with H2(15)O positron emission tomography. AJNR Am J Neuroradiol 24:419–426
Kouchi T, Tanabe Y, Smit EJ et al (2020) Clinical application of four-dimensional noise reduction filtering with a similarity algorithm in dynamic myocardial computed tomography perfusion imaging. Int J Cardiovasc Imaging 36:1781–1789. https://doi.org/10.1007/s10554-020-01878-6
Tsuneta S, Oyama-Manabe N, Kameda H et al (2020) Improvement of image quality on low-dose dynamic myocardial perfusion computed tomography with a novel 4-dimensional similarity filter. Medicine (Baltimore) 99:e20804. https://doi.org/10.1097/MD.0000000000020804
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Ohno Y, Yui M, Yoshikawa T et al (2021) 3D Oxygen-Enhanced MRI at 3T MR System: comparison with thin-section CT of quantitative capability for pulmonary functional loss assessment and clinical stage classification of COPD in Smokers. J Magn Reson Imaging 53:1042–1051. https://doi.org/10.1002/jmri.27441
Ohno Y, Yui M, Chen Y, Kishida Y, Seki S, Yoshikawa T (2019) Gadolinium-based blood volume mapping from MRI With ultrashort TE versus CT and SPECT for predicting postoperative lung function in patients with non-small cell lung cancer. AJR Am J Roentgenol 212:57–66. https://doi.org/10.2214/ajr.18.20095
Ohno Y, Koyama H, Matsumoto K et al (2011) Differentiation of malignant and benign pulmonary nodules with quantitative first-pass 320-detector-row perfusion CT versus FDG PET/CT. Radiology 258:599–609. https://doi.org/10.1148/radiol.10100245
Ohno Y, Nishio M, Koyama H et al (2013) Comparison of quantitatively analyzed dynamic area-detector CT using various mathematic methods with FDG PET/CT in management of solitary pulmonary nodules. AJR Am J Roentgenol 200:W593-602. https://doi.org/10.2214/ajr.12.9197
Sasaki M, Kudo K, Ogasawara K, Fujiwara S (2009) Tracer delay-insensitive algorithm can improve reliability of CT perfusion imaging for cerebrovascular steno-occlusive disease: comparison with quantitative single-photon emission CT. AJNR Am J Neuroradiol 30:188–193. https://doi.org/10.3174/ajnr.A1274
Kudo K, Boutelier T, Pautot F et al (2014) Bayesian analysis of perfusion-weighted imaging to predict infarct volume: comparison with singular value decomposition. Magn Reson Med Sci 13:45–50
Sasaki M, Kudo K, Boutelier T et al (2013) Assessment of the accuracy of a Bayesian estimation algorithm for perfusion CT by using a digital phantom. Neuroradiology 55:1197–1203. https://doi.org/10.1007/s00234-013-1237-7
Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med 36:715–725. https://doi.org/10.1002/mrm.1910360510
Sakai Y, Delman BN, Fifi JT et al (2018) Estimation of ischemic core volume using computed tomographic perfusion. Stroke 49:2345–2352. https://doi.org/10.1161/STROKEAHA.118.021952
Campbell BC, Christensen S, Levi CR et al (2011) Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke 42:3435–3440. https://doi.org/10.1161/STROKEAHA.111.618355
Funding
This work was supported by Canon Medical Systems Corporation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Ichiro Nakahara, and analysis was performed by Satomu Hanamatsu. The first draft of the manuscript was written by Kazuhiro Murayama, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This retrospective study was approved by the Institutional Review Board of Fujita Health University (Toyoake, Japan).
Consent to participate
Written informed consents were waived for all subjects in this retrospective study according to the approval of the local ethics committee (Fujita Health University, Toyoake, Japan).
Consent for publication
Written informed consents were waived for all subjects in this retrospective study according to the approval of the local ethics committee (Fujita Health University, Toyoake, Japan).
Conflict of interest
Kazuhiro Murayama, Kazuhiro Katada, Yoshiharu Ohno, and Hiroshi Toyama have received a research grant from Canon Medical Systems Corporation, which also supported this work technically. Ewoud J. Smit has received speaker honorarium from Canon Medical Systems Corporation. Mathias Prokop has received personal fees from Bracco, Bayer, Canon Medical Systems, and Siemens Healthineers and grants from Canon Medical Systems and Siemens Healthineers. Kazuhiro Katada is consultant to Canon Medical Systems Corporation. Yoshihiro Ikeda and Kenji Fujii are employees of Canon Medical Systems. The other authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murayama, K., Smit, E.J., Prokop, M. et al. A Bayesian estimation method for cerebral blood flow measurement by area-detector CT perfusion imaging. Neuroradiology 65, 65–75 (2023). https://doi.org/10.1007/s00234-022-03013-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03013-9